In the present era, infertility is one of the major issues which restricts many couples to have their own children. Infertility is the inability to achieve a clinical pregnancy after regular unprotected sexual intercourse for the period of one year or more. Various factors including defective male or female germ cell development, unhealthy and improper lifestyles, diseases like cancer and associated chemo-or-radiation therapies, congenital disorders, etc., may be responsible for infertility. Therefore, it is highly important to understand the basic concepts of germ cell development including primordial germ cell (PGC) formation, specification, migration, entry to genital ridges and their molecular mechanisms, activated pathways, paracrine and autocrine signaling, along with possible alteration which can hamper germ cell development and can cause adversities like cancer progression and infertility. Knowing all these aspects in a proper way can be very much helpful in improving our understanding about gametogenesis and finding possible ways to cure related disorders.
- oocytes
- Female Germ Cell
1. Female Germ Cell: In Vivo Developmental Stages (Primordial Germ Cells to Oocyte)
1.1. Primordial Germ Cell Development
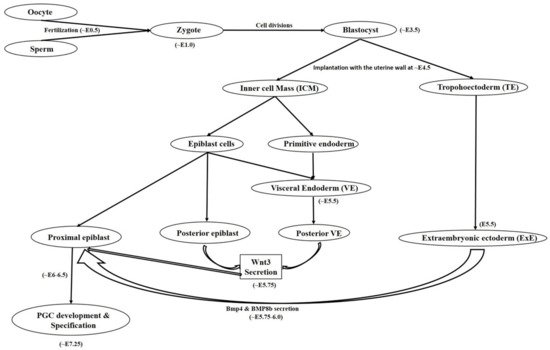
1.2. Primordial Germ Cell Migration
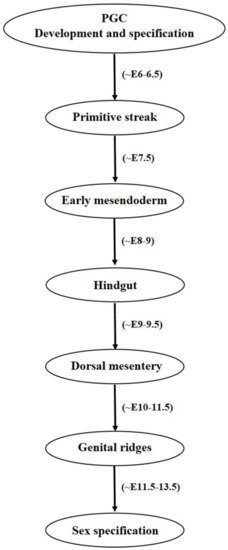
2. Functional Efficacy of the Female Germ Cells
3. Developmental Impairments and Associated Abnormalities
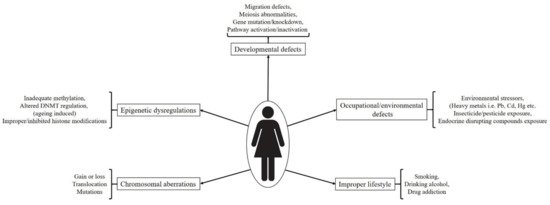
3.1. Chromosomal Aberrations
3.2. Spontaneous Genetic and Epigenetic Regulations
3.3. External/Environmental Factors and Incorrect Lifestyle Associated Defects/Exposure to Toxicants In Utero
3.3.1. Chemical Intoxicants
3.3.2. Smoking, Alcoholism and Drug Abuse
This entry is adapted from the peer-reviewed paper 10.3390/ijms22041979
