Multi-factors, such as anorexia, activation of renin-angiotensin system, inflammation, and metabolic acidosis, contribute to malnutrition in chronic kidney disease (CKD) patients. Most of these factors, contributing to the progression of malnutrition, worsen as CKD progresses. Protein restriction, used as a treatment for CKD, can reduce the risk of CKD progression, but may worsen the sarcopenia, a syndrome characterized by a progressive and systemic loss of muscle mass and strength. The concomitant rate of sarcopenia is higher in CKD patients than in the general population. Sarcopenia is also associated with mortality risk in CKD patients. Thus, it is important to determine whether protein restriction should be continued or loosened in CKD patients with sarcopenia.
- malnutrition
- protein energy wasting (PEW)
- sarcopenia
1. Introduction
Nutritional adverse derangement is often observed in patients with chronic kidney disease (CKD), and common in advanced CKD and dialysis patients. The International Society of Renal Nutrition and Metabolism (ISRNM) defines protein energy wasting (PEW) as a “the state of decreased body protein and fat masses” [1]. The pathogenesis of PEW in CKD is multifaceted. Decreased protein and energy intake due to dietary restriction or anorexia, increased protein catabolism due to activation of renin-angiotensin system or hyperparathyroidism, decreased anabolism due to insulin resistance, chronic inflammation, metabolic acidosis, and hormonal imbalances have been reported to be associated with PEW [2] as well as sarcopenia [3] (Figure 1). These two concepts share the same criteria and have similar causes and outcomes, but they are defined differently [4]. PEW focuses on protein and energy loss associated with inflammation, whereas sarcopenia focuses on muscle mass and strength loss associated with aging. CKD patients often belong to both conditions to varying degrees.
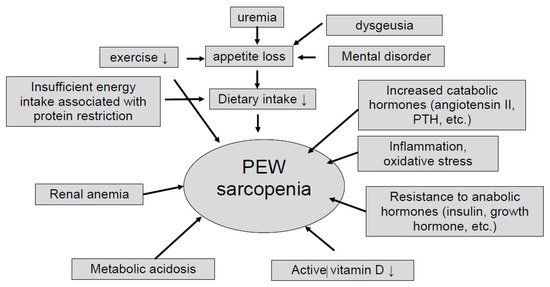
Dietary protein intake gradually decreases during the progression of kidney injury, even in the CKD patients with minimal dietary intervention [5]. This trend was similarly observed for urinary creatinine excretion, a marker of muscle mass [5]. Food intake progressively and spontaneously decreases with decline of renal function [6]. Infusion of angiotensin II in rats was shown to induce skeletal muscle wasting via proteolysis [7,8], and in turn, angiotensin converting enzyme inhibitor [9] or angiotensin receptor blocker [10] preserved muscle strength. Parathyroid hormone (PTH) was reported to drive adipose tissue browning and malnutrition via PTH receptor in fat tissue [10]. Systemic inflammation, including elevated cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, and so on, is often observed in CKD patients, and this tendency becomes more pronounced as the CKD stage progresses [11]. The inflammation in uremic milieu induces cardiovascular diseases and malnutrition [12,13]. Metabolic acidosis increases protein catabolism via up-regulation of ubiquitin-proteasome system in CKD patients [14,15]. Anemic patients were more frequently malnourished or at risk of malnutrition, and albumin levels are strongly associated with anemia in the elderly [16]. Vitamin D is associated with muscle weakness in older people [17], and pre-dialysis CKD patients [18].
As mentioned above, many factors contribute to malnutrition in CKD. Most of these factors, such as hyperparathyroidism, activation of renin-angiotensin system, metabolic acidosis, and insulin resistance, worsen as CKD progresses. Thus, nutritional condition in CKD patients deteriorates as CKD progresses. In fact, protein energy wasting (PEW) assessed by clinical global assessment was observed in 2% of CKD stage G1-2, 16% of CKD G3-4, 31% of CKD G5 without dialysis, and 44% of CKD G5D [19]. On the other hand, protein restriction is used as a treatment for CKD, but it may lead to sarcopenia, assessed by loss of muscle strength or mass [20]. Energy-adjusted protein intake was associated with three-year changes in lean mass body. Participants in the highest quintile of protein intake lost approximately 40% less lean mass than did those in the lowest quintile of protein intake [20].
2. CKD with Sarcopenia and Protein Restriction
End-stage kidney disease (ESKD) and death/cardiovascular death (mortality) are both important as the outcomes of CKD patients, and protein restriction is mainly used to improve the outcome of the former. In elderly CKD patients, the mortality risk is higher than the risk of ESKD [37]. Many of CKD patients with sarcopenia have been treated with protein restriction as a standard therapy. Kidney Disease Quality Initiative—National Kidney Foundation (KDOQI-NKF) guidelines for nutrition in CKD recommends a protein intake of 0.6 to 0.8 g/kg/day for patients with CKD in stages 3 to 5 with an energy intake of 30 kcal/kg/day [38]. The PROT-AGE Study Group recommends a protein intake of 0.8 g/kg/day and >0.8 g/kg/day for the elderly CKD patients with GFR < 30 mL/min and 30 to 60 mL/min, respectively [39]. However, in the case of muscle wasting such as sarcopenia, sufficient energy (30 kcal/kg/day) and protein (0.8–1.0 g/kg/day) are recommended for nutritional needs [4]. Some CKD patients with sarcopenia are at high risk for CKD progression to end-stage renal failure, while others have worsening sarcopenia and are at high risk of shortened life expectancy. In CKD patients with sarcopenia, different decisions (protein intake or protein restriction) need to be made against the dual outcomes of the progression of renal damage and the progression of sarcopenia. If the risk of end-stage renal failure is high, protein restriction is necessary, and if the risk of worsening sarcopenia is high, protein restriction should be loosened. However, such criteria are not clear. Thus, it is important to decide whether protein restriction should be continued or loosened in CKD patients with sarcopenia. CKD patients classified to stage G4 to G5 are belong to the extremely high risk group of renal replacement therapy, and prone to complications such as accumulation of uremic toxins, electrolyte abnormalities, and metabolic disorders [40,41]. Protein restriction can reduce the risk of ESKD in CKD, especially in patients with a GFR < 30 mL/min/1.73 m2, but does not increase the risk of death [42], suggesting that protein restriction should be considered a priority for patients with CKD stage G4 to G5.
The relative risk of ESKD for CKD patients with stage G3 varies greatly depending on the urinary protein level and rate of eGFR decline. Therefore, the risk of ESKD should be assessed in each individual case to determine whether protein restriction should be continued or loosened in CKD patients with stage G3. It has been reported that the risk of ESKD is low in CKD patients with A1 and A2 severity categories, and the mortality risk is higher than the risk of ESKD in cases with urinary protein levels <0.5 g/day [43]. On the contrary, it has been reported that proteinuria >1.0 g/gCr [44] or albuminuria > 1.0 g/gCr [45] is associated with a higher risk of ESKD.
With regard to the rate of eGFR decline, it has been reported that CKD patients with an eGFR decline >3.0 mL/min/1.73 m2/year have a higher risk of ESKD than those with a lower eGFR decline [46,47]. In a meta-analysis showing that protein restriction suppresses the rate of GFR decline [48], 12 of the 15 studies showed that the rate of GFR decline was greater than 3.0 mL/min/1.73 m2/year, suggesting that protein restriction may be effective in patients with a faster rate of eGFR decline. Thus, it is reasonable to consider prioritizing protein restriction in CKD patients with stage G4 to G5, but loosening protein restriction in CKD stage G3 patients with proteinuria <0.5 g/day, and rate of eGFR decline <3.0 mL/min/1.73 m2/year.
3. Effect of Increased Protein Intake for CKD Patients with Sarcopenia
4. Increased Protein Intake and Exercise for CKD Patients with Sarcopenia
In a systematic review and meta-analysis of three RCTs of elderly people with sarcopenia, exercise therapy improves limb skeletal muscle mass, normal walking speed, maximal walking speed, and knee extension muscle strength compared with dietary intervention or health education [52], suggesting that exercise therapy is effective in improving sarcopenia. In addition, there are several reports that exercise therapy, including resistance exercise, prolonged six-minute walking distance [53], increases thigh cross-sectional area, volume, and knee extension muscle strength [54], and increases muscle fiber area and upper and lower limb muscle strength [55,56]. Furthermore, in a 12-week RCT in CKD patients with stages G3b to G5, including frailty patients, the combination of resistance exercise and aerobic exercise increases muscle mass and strength compared with aerobic exercise alone [57], and it is important to note that there are no significant changes in renal function in both groups. These results suggest that exercise therapy is effective in improving muscle mass and strength in elderly patients with sarcopenia, and that the combination of exercise therapy and diet therapy is more effective than exercise therapy alone in improving sarcopenia in elderly patients. On the other hand, a large increase in protein intake may worsen the renal function in CKD patients. Therefore, it is considered safer to increase protein intake gradually in CKD. Although the amount of energy consumed during exercise therapy varies widely among individuals, total energy requirements are also expected to increase, so energy intake should be adjusted accordingly.
This entry is adapted from the peer-reviewed paper 10.3390/nu13041205
