Hepatocellular carcinoma (HCC) accounts for 80–90% of liver cancers and represents the third leading cause of cancer mortality worldwide. Over the past two decades, the incidence of HCC has doubled in many countries including Europe and United States [
1]. While approximately 75% of HCCs were associated with hepatitis B or C infection, other major risk factors including aflatoxin B1 (AFB1) exposure, nonalcoholic fatty liver disease (NAFLD), chronic alcohol consumption and obesity are now gaining more and more relevance [
2]. Among treatment options, surgical resection and percutaneous ablation are considered “curative” modalities; however, they can be performed in a restricted fraction of patients, particularly those detected at early stages. When diagnosed at, or progressed to, intermediate-stage, the standard of care is transarterial chemoembolization [
3], while large tumors or HCC with vascular invasion can be considered for radioembolization with selective internal radiation treatment (SIRT). When diagnosed at advanced stages or in the presence of extra-hepatic spread, only systemic treatments can be administered. Sorafenib, the first oral multikinase inhibitor (MKI) entering the treatment of HCC, has been the standard of care for almost ten years [
4]. It inhibits angiogenesis and cell proliferation by targeting platelet-derived growth factor receptor (PDGF-R), vascular endothelial growth factor receptor (VEGFR-2/3), c-Kit, Flt3 and Raf kinases involved in the MAPK/ERK pathway [
5].The second tyrosine kinase inhibitor to be approved for the first line treatment of advanced HCC is lenvatinib, based on its non-inferiority to sorafenib [
6]. In the setting of second line treatments, regorafenib and cabozantinib were approved for patients with HCC progression and ramucirumab for patients previously treated with sorafenib, with a serum AFP (Alpha-Fetoprotein) level ≥400 ng/mL [
7]. Beside these molecularly-targeted agents, immuno-oncology has opened the way to a novel class of drugs modulating the expression of Immune Check Point Inhibitors (ICPI), whose aberrant expression results in the immune escape of cancer cells [
8]. Programmed cell death protein 1 (PD1), Programmed Death-Ligand 1 (PDL1), Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA4) are the most targeted ICPI in HCC proving to be more effective than Tyrosine Kinase Inhibitors (TKI), especially in combination regimens [
9]. The most interesting results have been obtained so far with the association of Atezolizumab and Bevacizumab [
10], however other promising combinations are under clinical investigation. Large clinical trials are however still needed for validation of these effects and for the identification of biomarkers helping the allocation of patients to the most effective treatment option in a personalized perspective. In addition, beside the fraction of patients who are non-responders to ICPIs, others may experience adverse events which prevent the prosecution of treatment. Unfortunately, the molecular classification of HCC has not entered clinical practice so far; thus, genetic and epigenetic factors driving response or resistance to each treatment are still poorly understood.
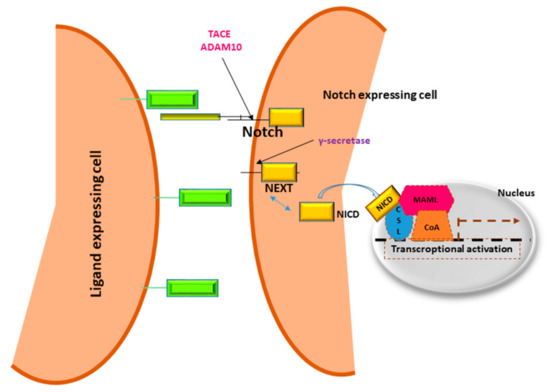
Deregulation of multiple molecular signaling pathways such as Wnt/β-catenin, Ras mitogen-activated protein kinase (Ras/Raf/MAPK), phosphatidylinositol 3-kinase (PI3K), AKT/mammalian target of rapamycin (mTOR), Janus kinase (Jak)-signal transducer activator of transcription factor (Stat) (JAK/STAT) and the Hippo signaling pathway are essential for HCC development and progression [
11]. Understanding the critical genes and signaling molecules in different HCC subgroups will help to develop tailored therapeutic strategies. Inhibition of a single signaling cascade may induce feedback activation of other pathways; hence, combination of different molecularly targeted agents is expected to show synergistic activity. Notch signaling modulates the development and functions of several immune cell lineages. Among these, Notch1 and Dll4 interaction participates in T cells’ lineage commitment, while Notch2-Dll1 interaction contributes to the development of marginal zone B cells [
12]. In the peripheral T and B cell compartment, Notch signaling activates T cells’ proliferation and cytokine production, which, in line, are down-regulated by GSI-mediated inhibition of Notch. Similarly, Notch1 and Notch2 activation promotes naive CD8+ T cells to cytotoxic T lymphocytes, which, in turn, drive the antitumoral response and are the targets of ICPIs.
Investigations on Notch signaling in T cells functions might lead to a more effective avenue for combined intervention in cancer treatment.
The aim of this review is to discuss recent advances in molecular mechanisms involved in Notch signaling regulation that could represent new challenges for HCC therapy. Studies described in this review might contribute to the identification of key molecules responsible for Notch pathway regulation that could represent new therapeutic targets.