Photosynthesis is the process that transforms light energy into chemical energy and thus forms the basis of all life on Earth, ranging from photosynthetic bacteria to higher plants. In plants, photosynthesis takes place in chloroplasts. Since chloroplasts contain approximately 80% of the total leaf nitrogen (N) [
15], translocation of N compounds from chloroplasts in older leaves (source organs) to new developing tissues (sink organs) is one of the most crucial events during the process of leaf senescence. In addition, increased expression of senescence-associated genes (SAGs) is promoted by the decline in photosynthetic activity and photosynthesis-related gene expression [
16]. Thus, the expression of SAGs during senescence is probably greatly affected by the status of photosynthesis, and the nucleus may obtain the information about the status of photosynthesis through signals produced in chloroplasts. The transport of such retrograde signals from chloroplasts to the nucleus to increase the expression of SAGs is still largely unknown, but several important clues have been found in recent studies.
2.1. Chloroplast-Produced Reactive Oxygen Species (ROS) Affect Leaf Senescence
Reactive oxygen species (ROS) are highly reactive molecules generated by the reduction of ground-state molecular oxygen (
3O
2) through the acceptance of an electron pair. In plants, the major forms of ROS include superoxide anion (O
2−), hydroxyl radical (·OH), hydrogen peroxide (H
2O
2), and singlet oxygen (
1O
2). Among these, O
2−, ·OH, and H
2O
2 are produced in most subcellular compartments, while the highly reactive
1O
2 is uniquely produced in chloroplasts, which are the primary source of ROS under light conditions [
17]. ROS production in chloroplasts is tightly associated with light-dependent photosynthetic reactions. During photosynthesis,
1O
2 is produced mainly within photosystem II (PSII) in thylakoid membranes because of the reaction between the triplet state of chlorophyll molecule and
3O
2 [
17]. On the other hand, the reduction of
3O
2 by PSI generates O
2−, which is immediately converted into H
2O
2 on the stromal side of the thylakoid membranes either spontaneously or through the action of superoxide dismutases (SODs) [
18]. The production of ROS is drastically increased under unfavorable environmental conditions, such as excess light, drought, and high temperature [
19]. In addition, it is well known that the production of ROS, especially H
2O
2, is significantly increased in leaves during senescence [
20], which is accompanied by a significant reduction in the abundance of light harvesting complex II (LHCII) and other photosystem proteins. Consequently, the captured light energy is in excess for the remaining photosynthetic units, leading to the production of a large amount of ROS even under moderate light conditions. In addition, the dismantling of LHCII subunits releases a large number of chlorophyll molecules and their degradation products. It is well known that several chlorophyll biosynthesis intermediates, including protoporphyrin IX and protochlorophyllide, act as photosensitizers that produce high amounts of
1O
2 under light conditions [
21,
22]. Similarly, several chlorophyll degradation products, such as 7-hydroxymethyl chlorophyll
a, pheophorbide
a, and red chlorophyll catabolite, also act as photosensitizers [
23,
24,
25] ().
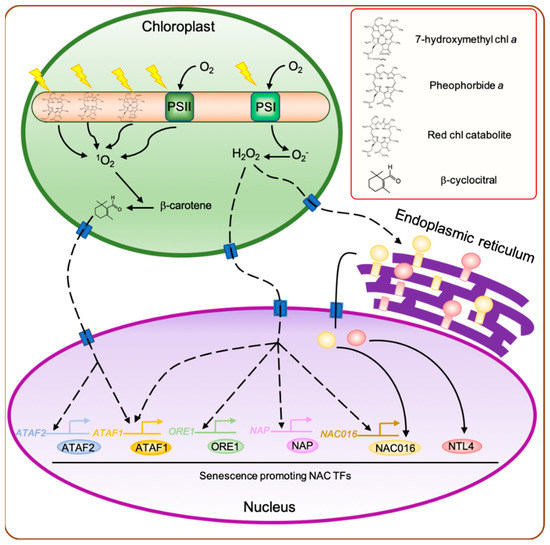
Figure 1. Regulatory network of leaf senescence induced by chloroplast-derived retrograde signaling. During the senescence phase, the abundance of photosystem proteins decreases significantly. Consequently, the captured light energy exceeds the amount needed by the remaining photosynthetic units, leading to the production of a large amount of O2− by photosystem I (PSI) and 1O2 by PSII. In addition, the dismantling of light harvesting complex II (LHCII) proteins releases a large number of chlorophyll molecules and their degradation products, such as 7-hydroxymethyl chlorophyll a, pheophorbide a, and red chlorophyll catabolite, which act as photosensitizers, producing high amounts of 1O2 in the presence of light. The 1O2 generated in chloroplasts promotes the cleavage of β-carotene to produce β-cyclocitral, which then acts as a retrograde signal between the chloroplast and nucleus to activate the expression of 1O2-responsive genes, including senescence-associated Arabidopsis thaliana activating factor (ATAF) subfamily no apical meristem/ATAF1,2/cup-shaped cotyledon (NAC) genes, ATAF1 and ATAF2. O2− generated by PSI is rapidly dismutated into H2O2, which acts as a signaling molecule to alter the expression of H2O2-responsive genes, including ATAF1, ORESARA1 (ORE1), NAC-like, activated by AP3/PI (NAP), and ANAC016. In addition, H2O2 generated in the chloroplasts also triggers the translocation of membrane-bound NAC transcription factors (TFs), such as ANAC016 and NAC with transmebrane motif 1-LIKE4 (NTL4), from the endoplasmic reticulum to the nucleus. Dashed arrowheads indicate the indirect activation of genes. chl, chlorophyll.
For many years, it has been proposed that ROS generated in chloroplasts are involved in the promotion of leaf senescence (reviewed in [
26,
27]). Among these ROS, H
2O
2 plays a crucial role in the regulation of leaf senescence. Since H
2O
2 can easily cross cellular membranes [
28], it can be transmitted as a signaling molecule from the chloroplast to the nucleus. Transcriptome analyses of Arabidopsis revealed that several genes upregulated during leaf senescence are also upregulated by H
2O
2 treatment [
29,
30]; for example, no apical meristem/ATAF1,2/cup-shaped cotyledon (NAC) TF-encoding genes, such as
Arabidopsis thaliana activating factor1 (ATAF1)/ANAC002 [
31],
ANAC016 [
32],
NAC-like, activated by
AP3/PI (NAP)/ANAC029 [
33],
JUNGBRUNNEN1 (JUB1)/ANAC042 [
34], and
ORESARA1 (
ORE1)
/ANAC092 [
35], which have been characterized as key regulators of leaf senescence (). Among these,
JUB1 acts as a negative regulator of leaf senescence, while the other four
NAC genes promote leaf senescence.
H
2O
2 is also one of the triggers that induce the translocation of membrane-bound NAC TFs from the endoplasmic reticulum (ER) membrane to the nucleus. Several membrane-bound NAC TFs are activated by H
2O
2 generated in mitochondria under environmental stresses [
36,
37]. The induction of nuclear gene expression by treatment with methyl viologen, which accepts electrons from PSI via ferredoxin and leads to the production of a large amount of O
2− in chloroplasts, is strongly inhibited in the knockout mutant of
ANAC017, an Arabidopsis membrane-bound NAC TF gene [
38], indicating that H
2O
2 generated in chloroplasts is also important for the activation of membrane-bound NAC TFs. In Arabidopsis and rice (
Oryza sativa L.), several membrane-bound NAC TFs including ANAC016 [
32], NAC WITH TRANSMEMBRANE MOTIF 1-LIKE4 (NTL4)/ANAC053 [
39], and ONAC054 [
40] have been shown to promote the initiation of leaf senescence. Thus, H
2O
2 generated in chloroplasts during the senescence phase may modulate the gene expression partially via the activation of senescence-associated membrane-bound NAC TFs ().
O
2− also modulates the expression of SAGs. In Arabidopsis seedlings treated with methyl viologen, an O
2−-specific propagator, several genes encoding senescence-associated TFs including
ATAF1,
WRKY6 [
41], and
WRKY22 [
42] were strongly upregulated [
43]. WRKY6 and WRKY22 antagonistically regulate leaf senescence; WRKY6 acts as a negative regulator, while WRKY22 acts as an enhancer of leaf senescence. In contrast with H
2O
2, O
2− is a short-lived ROS and cannot cross the chloroplast membrane [
44]. Thus, O
2− probably generated in chloroplasts cannot serve as a signaling molecule.
Compared with H
2O
2 and O
2−,
1O
2 is a highly reactive molecule and consequently a more potent oxidizing agent than the other ROS [
45]. In leaf tissues, in particular,
1O
2 is required for most of the lipid peroxidation reactions. Lipid peroxidation promotes the generation of free radicals, which accelerate senescence [
46].
1O
2 also gives rise to a signal that affects the expression of nuclear genes, similar to the other two ROS. Op den Camp et al. (2003) investigated the effect of
1O
2 on nuclear gene expression using Arabidopsis
flu mutant, which shows greater accumulation of protochlorophyllide than the wild type. Since protochlorophyllide acts as a photosensitizer, the
flu mutant generates a large amount of
1O
2 when transferred from dark to light conditions [
22]. In dark-grown
flu mutant seedlings, incubation under light for 2 h significantly upregulated the expression of several stress-responsive genes including
ABSCISIC ACID (
ABA)
INSENSITIVE 1 (
ABI1), which encodes a protein phosphatase that acts as a negative regulator of ABA signaling [
47], and
1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE 4 (
ACO4), which encodes an ethylene biosynthesis enzyme [
48]. In addition to their roles in abiotic stress response, ABA and ethylene are also known to promote leaf senescence [
49]. Thus,
1O
2 may affect the transcriptional regulatory networks of leaf senescence by modulating the expression of stress response and leaf-senescence-related genes.
2.2. State of Photosystem Proteins Determines the Initiation of Leaf Senescence
Several studies indicate that the state of photosystem proteins affects the initiation of leaf senescence. Chlorophyllide a oxygenase (CAO) is a chlorophyll biosynthesis enzyme that catalyzes two reactions: the conversion of chlorophyll
a into 7-hydroxymethyl chlorophyll
a and the subsequent transformation of 7-hydroxymethyl chlorophyll
a into chlorophyll
b [
50]. The N-terminal domain of CAO proteins, which is required for their destabilization, is conserved among higher plants [
51]. Transgenic Arabidopsis plants overexpressing N-terminal domain-truncated CAO overaccumulated chlorophyll
b and stayed green during the senescence phase under both light and dark conditions [
52]. Moreover, in these transgenic plants, the abundance of chlorophyll
b in the antenna proteins of PSI and PSII increased significantly, and chlorophyll
b was also incorporated into CP43, a PSII core protein, which otherwise binds only to chlorophyll
a [
53]. One of the possible explanations for the stay-green phenotype of these transgenic plants is the stabilization of photosystem proteins due to the increased incorporation of chlorophyll
b in photosystem core complexes. Similar to the
CAO overexpression lines, Arabidopsis and rice knockout mutants of
NON-YELLOW COLORING1 (
NYC1), which encodes chlorophyll
b reductase, also showed the stay-green phenotype with highly retaining of antenna proteins of photosystems, as well as the structure of grana thylakoid, during senescence [
54,
55]. Rice
delayed yellowing1 (
dye1) mutants, in which the pigment-binding function of one of the LHCI subunits (Lhca4) was impaired, exhibited prolonged greenness during the senescence phase [
56]. In
dye1 mutant plants, although the accumulation of other Lhca proteins (Lhca1, Lhca2, and Lhca3) was similar to that in the wild type, Lhcb1 (a subunit of LHCII) showed high accumulation [
56]. Since the chlorophyll
b content of the
dye1 mutant increased because of the increase in the chlorophyll
b-binding capacity of LHCII proteins, this result also supports the hypothesis that the abundance of chlorophyll
b is positively correlated with the stability of PSI and PSII during senescence. Additionally, a large number of SAGs were differentially expressed between
CAO-overexpressing and wild-type Arabidopsis plants [
52]. Thus, the stabilization of photosystem proteins, partially by chlorophyll
b, is one of the key factors affecting the abundance of signaling molecules required for the induction of leaf-senescence-related transcriptional-regulatory networks.
2.3. Potential Role of Chlorophyll and Caroteinoid Degradation Products as Retrograde Signaling Molecules in the Regulation of Leaf Senescence
Chlorophyll biosynthesis and degradation intermediates have the potential to produce ROS as photosensitizers, as described above. However, according to several other studies, some chlorophyll intermediates can mediate retrograde signaling from the chloroplasts to the nucleus. It has been shown that Mg-protoporphyrin IX (Mg-proto IX) can act as a negative regulator of nuclear genes associated with photosynthesis. Exogenous application of Mg-proto IX to Arabidopsis protoplasts has been shown to greatly inhibit the expression of Lhcb [
57]. In addition, Mg-proto IX can bind to heat-shock protein 90 (HSP90) to decrease its ATPase activity, which subsequently decreases the expression of genes associated with photosynthesis, probably via the regulation of LONG HYPOCOTYL5 (HY5) [
58]. Considering the function of Mg-proto IX, it is also probable that chlorophyll degradation intermediates, such as 7-hydroxymethyl chlorophyll
a, pheophytin
a, and pheophorbide
a, act as signaling molecules. Indeed, several genes associated with chlorophyll degradation were upregulated in transgenic Arabidopsis plants overexpressing
PHEOPYTINASE (
PPH) [
59], which encodes a chlorophyll degradation enzyme that catalyzes the conversion of pheophytin
a into pheophorbide
a [
60]. Similarly, STAY-GREEN1 (SGR1), a chlorophyll degradation enzyme that converts chlorophyll
a into pheophytin
a [
61], also affects the expression of genes associated with leaf senescence. For example, when
SGR1 was overexpressed via the dexamethasone (DEX)-inducible system, genes associated with chlorophyll degradation, such as
PPH,
SGR2 [
61],
NYC1 [
55], and
PAO [
24], and other SAGs, such as
ORE1, were significantly upregulated [
62]. These results indicate that the accumulation of chlorophyll degradation intermediates or changes in the activity of chlorophyll degradation enzymes may lead to retrograde signaling between the chloroplast and nucleus to activate the transcriptional regulatory networks involved in leaf senescence. The other possibility is that chlorophyll degradation intermediates themselves act as signaling molecules.
Ramel et al. (2012) revealed that in Arabidopsis, high light-induced
1O
2 promotes the cleavage of β-carotene, and the resulting breakdown products including β-cyclocitral mediate
1O
2-related retrograde signaling to modulate the expression of genes associated with photooxidative stress [
63]. Among these genes, four
NAC genes,
ATAF1,
ANAC032,
ATAF2/ANAC081, and
ANAC102, which encode a senescence-promoting NAC TF [
31,
64], are strongly upregulated by both β-cyclocitral and high light intensity [
65]. Given the elevated level of
1O
2 in chloroplasts during senescence phase, β-cyclocitral-mediated retrograde signaling may play a role in the regulation of leaf senescence