Betulinic acid (BA, 3β-hydroxy-lup-20(29)-en-28-oic acid) is a pentacyclic triterpene acid present predominantly in Betula ssp. (Betulaceae) and is also widely spread in many species belonging to different plant families. BA presents a wide spectrum of remarkable pharmacological properties, such as cytotoxic, anti-HIV, anti-inflammatory, antidiabetic and antimicrobial activities, including antiprotozoal effects.
- betulinic acid
- antiprotozoal activities
- triterpene acid derivatives
- antiplasmodial activity
- an-tileishmanial activity
1. Introduction
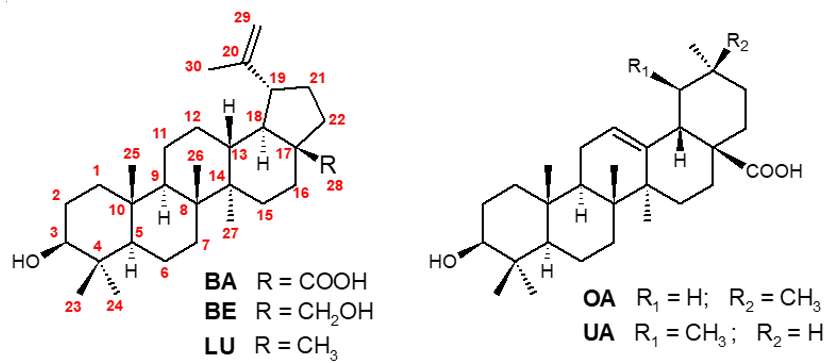
Betulinic acid (BA), namely 3β-hydroxy-lup-20(29)-en-28-oic acid, is a pentacyclic lupane-type triterpene (Figure 1) which was allegedly isolated for the first time from the bark of Cornus florida L. (Cornaceae) in 1939. At that time, its structural elucidation was based on data obtained by comparison with a series of synthetic derivatives [1]. Subsequently, BA was also found in the seeds of Zizyphus vulgaris Lam. (var. Spinosus Bunge, Rhamnaceae) [2], in the bark of Platanus acerifolia [3] and in the fibrous outer bark of Syncarpia laurifolia (currently var. glomulifera, Myrtaceae) [4]. For the proper identification of the isolates, the preparation of known chemical derivatives was also necessary. Curiously, it is assumed that BA was found even earlier in other species, but under different trivial names, such as gratiolone (isolated from Gratiola officinalis, Schropulariaceae) [5], platanol and/or platanine (from Platanus spp.) and an unidentified compound obtained from Cornus sanguinea L. and named as “platanoic acid” [3][5][6][7].
Figure 1. Structures of betulinic acid (BA), betulin (BE), lupeol (LU), oleanolic (OA) and, ursolic (UA) acids.
The name “Betulinic acid” has been given to this compound because of its prevalence in birch trees, which belong to the genus Betula, family Betulaceae, especially Betula alba, B. pubescens, B. platyphylla, B. maximowicziana and B. mandshurica. Additionally, although this family is the main natural source of BA, this triterpene is spread across many plant species belonging to the families Amaranthaceae, Ancistrocladaceae, Apocynaceae, Asteraceae, Chrysobalanaceae, Convolvuleaceae, Dichapetalaceae, Dilleniaceae, Dioncophyllaceae, Ebenaceae, Ericaceae, Fabaceae, Fagaceae, Lamiaceae, Loganiaceae, Melastomataceae, Moraceae, Myrtaceae, Onagraceae, Ramnaceae, Ranunculaceae, Rhamnaceae, Rosaceae, Rubiaceae, Trochodendraceae, Verbenaceae and Viscaceae [8]. Interestingly, this triterpene can be extracted in large quantities from Akania lucens Hook f. (Akaniaceae), Nerium oleander L. (Apocynaceae), Avicennia marina L. (Verbenaceae), Lemaireocereus spp. (Cactaceae), Arbutus menziesii Pursh. and Arctostaphylos uva-ursi (Ericaceae), Lavandula angustifolia var. officinalis (Lamiaceae), Nuytsia floribunda R. Br. (Loranthaceae), Tectona grandis L. f. (Verbenaceae), Davilla rugosa and other species of Dilleniaceae family (Dillenia, Wormia and Acrotrema). Thus, BA may be considered as a chemotaxonomic marker for these families/genera [9][10][11]. This compound seems to be biosynthesized from lupeol (LU, Figure 1) by the cytochrome CYP716A12, which has been characterized as a multifunctional enzyme showing lupeol 28-oxidase activities. The same cytochrome is responsible for the methyl C-28 oxidation of β- and α-amirins, providing, in addition to BA, the other common triterpenes olenanolic (OA) and ursolic (UA) acids (Figure 1) [12][13].
Only in 1976 the compound’s pharmacological importance begin to be evidenced. A study employing plant extracts containing BA exhibited high cytotoxicity and selectivity against lymphocytic leukemia P388 cells [14]. Sequentially, BA was identified as a melanoma-specific cytotoxic compound given that in vivo studies showed tumor growth inhibition without toxicity [15]. Since then, various studies with BA and derivatives as antitumor agents have been published [16][17]. Recent investigations have shown that the presence of the carboxyl function at C-28 is required for the cytotoxicity. This conclusion is supported in the fact of BA derivatives are usually significantly more potent than those derived from betulin (BE) (Figure 1) [18]. BA also shows potential as an anti-HIV agent, many of its derivatives, especially hybrids compounds, are highly effective against HIV [19][20]. Compared to azidothymidine (AZT), specific hybrid derivatives presented more potent or equipotent anti-HIV activities but displayed less cytotoxicity [21]. Additionally, numerous studies have been published describing a broad spectrum of other remarkable pharmacological properties for BA against fungi, bacteria, protozoa, diabetes, and inflammatory disorders [22][23][24]. Among all these potential applications for BA and its analogues, we have focused this review on their antiprotozoal activity, and particularly, on their application to tropical diseases transmitted by vectors.
Vector-borne tropical diseases are disorders that are prevalent in tropical and subtropical regions since the cold season in temperate climates often controls the insect populations (vectors) by forcing their hibernation. Among the most impactful tropical diseases, malaria, leishmaniases and trypanosomiases are globally widespread, with potentially harmful consequences if left untreated [25][26].
Malaria is a life-threatening protozoan disease caused by five Plasmodium species (P. falciparum, P. vivax, P. knowlesi, P. ovale and P. malariae) that are transmitted to people through the bites of infected female Anopheles mosquitoes. P. falciparum and P. vivax account for more than 95% of all human malaria infections and therefore represent a great threat and serious challenge to public health. In 2019, with an estimated 229 million cases and 409,000 deaths, nearly half of the world’s population was at risk of malaria. Most cases and deaths occur in sub-Saharan Africa and more severely affect children under 5 years old [27].
In turn, the leishmaniases are a group of diseases caused by protozoan parasites from more than 20 Leishmania species. These parasites are transmitted to humans by the bite of an infected female phlebotomine sandfly, and then three main forms of the disease may arise: cutaneous leishmaniasis (CL), the most common form; visceral leishmaniasis (VL), also known as kala-azar, the most severe form; and mucocutaneous leishmaniasis (MCL), the most disabling form of the disease. More than 1 billion people live in areas endemic for leishmaniasis and are at risk of infection. An estimated 30,000 new cases of VL and more than 1 million new cases of CL occur annually [28][29].
Finally, human trypanosomiasis comprises African trypanosomiasis and Chagas disease which are caused by protozoan parasites of the genus Trypanosoma. African trypanosomiasis is caused by either T. brucei gambiense or T. brucei rhodesiense and threatens some 65 million people in sub-Saharan Africa, especially in rural areas and populations disrupted by war or poverty. Alternatively, Chagas disease is caused by T. cruzi and is responsible for 21,000 deaths per year, occurring mainly in Latin America [29][30].
Since tropical diseases are not very attractive to pharmaceutical companies for the development of novel drugs [31], few options are available on the market to treat these protozoan disorders. Currently, the most effective antimalarial drugs available are chloroquine and artemisinin derivatives, whereas amphotericin B, paromomycin, miltefosine, pentamidine and sodium stibogluconate are the most important antileishmanial agents. Additionally, pentamidine, benznidazole and nifurtimox are the drugs of choice for the treatment of human trypanosomiasis. The problems related to the high economic and social costs of these drugs, along with their toxic effects and the emergence of drug resistance, point to the urgent need for novel antiprotozoal drugs [32].
2. Synthesis of BA from Betulin
In view of the highly valuable potential of BA in the development of novel drugs to treat protozoal and other impacting diseases, it is necessary to establish viable sources of this triterpene to allow for its use as a starting material for the preparation of new and improved bioactive analogues. Within this scenario, it is worth mentioning that promising results regarding the production of BA and derivatives by biocatalysis and other biotechnological strategies have been widely reported in the literature [33][34][35] and, therefore, they will not be covered in this review.
To the best of our knowledge, no total synthesis of BA has been reported yet. Although LU, its lupane C-28 methyl co-related triterpene, has been totally synthesized since the 1970s [36][37].
Currently, commercial production of BA substantially depends on traditional phytochemical extraction from birch bark [35], that yields around 0.025% w/w from dry material [38]. The structure related to BA, is the triterpene alcohol BE (Figure 1), which is much more abundant in birch trees (up to 34% of the dry barks) [39], but it is biologically less active [38][40] than BA. Therefore, BE could be employed as a starting material to easily prepare BA through semisynthesis, by oxidation of the primary hydroxyl group present at C-28.
The first reports of the synthesis of BA from BE were published in the late 1930s [1][41]. In this study, the authors used routes with a large number of steps and consequently, the overall yield was low.
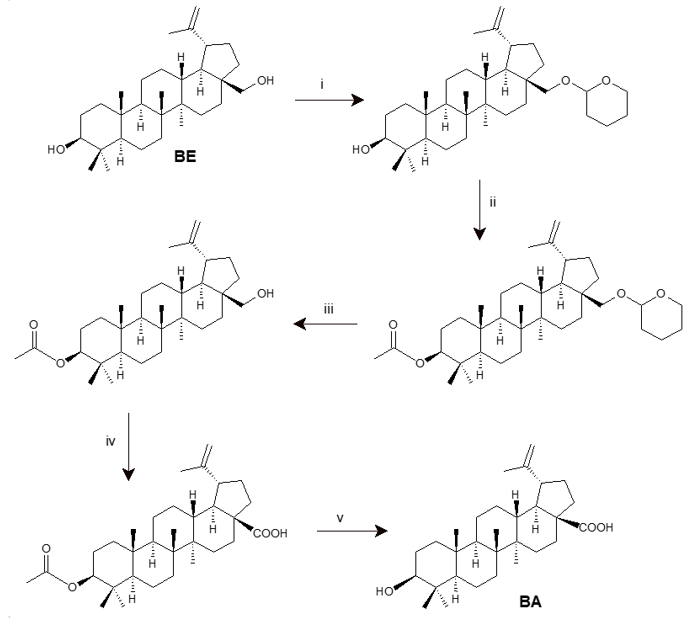
Decades later, a classical method was reported using protecting groups to selectively mask the C-3 OH group (secondary alcohol) to avoid its isomerization (Scheme 1). In this study, although this classical route involves five steps and uses chemical agents that are to both health and the environment (e.g., CrO3), BA could be advantageously produced with high stereoselectivity and a good overall yield [38].
Scheme 1. Synthesis of betulinic acid (BA) from betulin (BE) using protecting groups [38]. Reagents and conditions: (i) DHP/CH2C12/PPTS (95%); (ii) Ac2O/pyridine (87%); (iii) EtOH/PPTS (95%); (iv) CrO3/H2SO4/acetone (80%); (v) K2CO3/MeOH/H2O (88%).
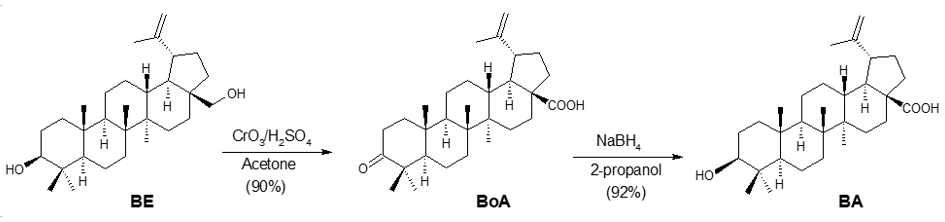
However, the most common methodology employed to synthesize BA from BE involves a direct Jones oxidation step (CrO3/H2SO4/acetone) to form first the intermediate betulonic acid (BoA), with a yield of 90%. Then, BoA is reduced with NaBH4/2-propanol to obtain, after recrystallization in hot methanol, pure BA. This way, a 3β-isomer yield of 92% is obtained (Scheme 2) [42].
Scheme 2. Synthesis of BA from BE through direct Jones oxidation.
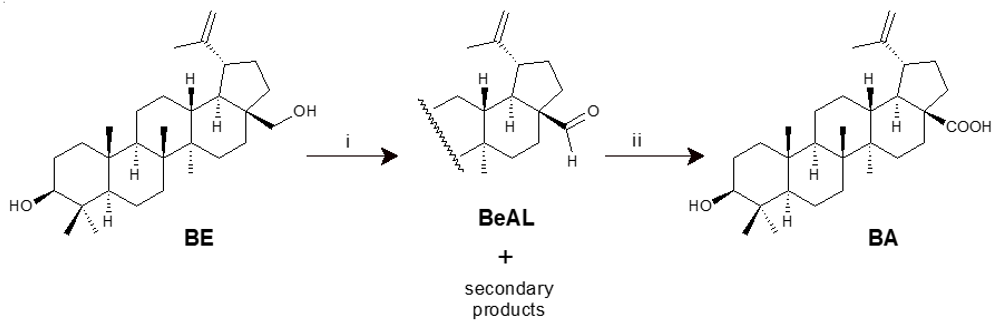
Recently, a two-step route using solid-supported chromium oxide and potassium permanganate has been suggested to improve BA synthesis [43]. The main step of this approach is the selective oxidation of the primary alcohol function of BE, accomplished with chromic oxide adsorbed on silica gel to obtain BeAL (betulinal) with a reasonable yield (64%). Next, the aldehyde derivative is oxidized to BA by potassium permanganate, resulting in a yield of 85% (Scheme 3).
Scheme 3. Synthesis of BA from BE via solid-supported CrO3 oxidation [43]. Reagents and conditions: (i) 2 eq. CrO3/SiO2 (1:10), toluene, 60 min (84%); (ii) 2 eq. KMnO4, acetone, 0 °C, 30 min (~100%).
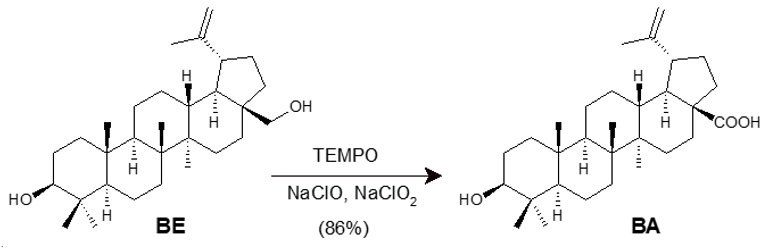
All of the above-mentioned approaches were designed to be small-scale preparations, employing more than one synthetic step with overall moderate to good yields. To overcome these limitations, Csuk and collaborators developed the short one-step route represented in Scheme 4 [40]. The method was based on the catalytic conversion of BE into BA, mediated by 4-acetamide-2,2,6,6-tetramethylpiperidine-1-oxyl (4-acetamide-TEMPO) in a reaction medium containing a mixture of NaClO and NaClO2 at 35 °C. It is worth mentioning that the simplicity of the synthetic route is the main advantage resulting from this study, in addition to the use of a cheap starting material and, especially, the possibility of obtaining BA on a larger scale, with a yield of 86% in just one step.
Scheme 4. One-step synthesis of BA from BE via 4-acetamido-TEMPO/NaClO/NaClO2.
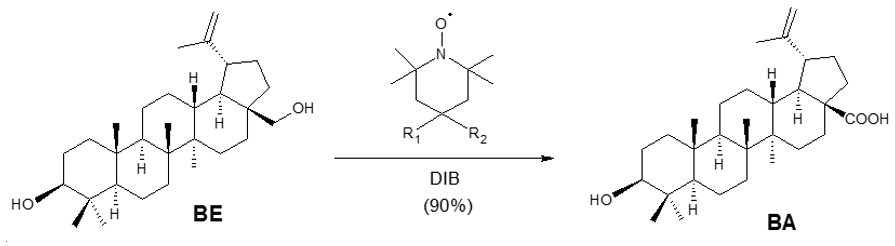
In a slightly different one-step method, a patented route also employing TEMPO-type catalysts, the hypervalent iodine reagent diacetoxy-iodobenzene (DIB) was used as the oxidizer [44]. The reaction occurs under mild conditions and is both economically and environmentally friendly, with a yield of up to 90% (Scheme 5).
Scheme 5. Synthesis of BA from BE via TEMPO-type catalysts/DIB.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26041081
References
- Robertson, A.; Soliman, G.; Owen, E.C. Polyterpenoid compounds. Part, I. Betulic acid from Cornus florida L. J. Chem. Soc. 1939, 1267–1273.
- Kawaguti, R.; Kim, K.W. The constituents of Zyzyphus vulgaris Lamark var. spinosus Bunge. II. Betulinic acid. Yakugaku Zasshi 1940, 60, 595–596.
- Bruckner, G.J.; Kovacs, J.; Koczka, I. Occurrence of betulinic acid in the bark of the plane tree. J. Chem. Soc. 1948, 948–951.
- Ralph, C.S.; White, D.E. Betulic acid from Syncarpia laurifolia. J. Chem. Soc. 1949, 3433–3434.
- Retzlaff, F. Ueber Herba gratiolae. Arch. Pharm. (Weinh.). 1902, 240, 561–568.
- Zellner, J.; Fajner, R. Wachsbestandteile in Hartriegelrinden (Cornaceae). Monatshefte 1925, 46, 611–630.
- Stabursvik, A. Occurrence of betulinic acid in Menyanthes trifoliata L. Acta Chem. Scand. 1953, 7, 446–447.
- Pai, S.R.; Joshi, R.K. Distribution of betulinic acid in plant kingdom. Plant. Sci. Today 2014, 1, 103–107.
- Pavanasasivam, G.; Sultanbawa, M.U.S. Betulinic acid in the dilleniaceae and a review of its natural distribution. Phytochemistry 1974, 13, 2002–2006.
- David, J.M.; Souza, J.C.; Guedes, M.L.S.; David, J.P. Estudo fitoquimico de Davilla rugosa: Flavonóides e terpenóides. Braz. J. Pharmacogn. 2006, 16, 105–108.
- Moghaddam, M.G.; Ahmad, F.B.H. Various botanical sources of betulinic acid: A review. Asian J. Chem. 2012, 24, 4843–4846.
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant. Cell Physiol. 2011, 52, 2050–2061.
- Zhou, C.; Li, J.; Li, C.; Zhang, Y. Improvement of betulinic acid biosynthesis in yeast employing multiple strategies. BMC Biotechnol. 2016, 16, 1–9.
- Trumbull, E.R.; Bianchi, E.; Eckert, D.J.; Wiedhopf, R.M.; Cole, J.R. Tumor inhibitory agents from Vauquelinia corymbosa (Rosaceae). J. Pharm. Sci. 1976, 65, 1407–1408.
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051.
- Zhang, D.; Xu, H.; Wang, L.; Li, Y.; Sun, P.; Wu, X.; Wang, G.; Chen, W.; Ye, W. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Harv. Bus. Rev. 2008, 86, 84–92.
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (Review). Mol. Med. Rep. 2016, 14, 4489–4495.
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.P.; Csuk, R. Betulinic acid derived amides are highly cytotoxic, apoptotic and selective. Eur. J. Med. Chem. 2020, 207, 112815.
- Huang, Q.; Chen, H.; Luo, X.; Zhang, Y.; Yao, X.; Zheng, X. Structure and Anti-HIV Activity of Betulinic Acid Analogues. Curr. Med. Sci. 2018, 38, 387–397.
- Wu, H.F.; Morris-Natschke, S.L.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385.
- Wang, Q.; Li, Y.; Zheng, L.; Huang, X.; Wang, Y.; Chen, C.H.; Cheng, Y.Y.; Morris-Natschke, S.L.; Lee, K.H. Novel Betulinic Acid-Nucleoside Hybrids with Potent Anti-HIV Activity. Acs Med. Chem. Lett. 2020, 11, 2290–2293.
- Ghaffari Moghaddam, M.; Bin, H.; Ahmad, F.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 03, 119–123.
- Ríos, J.L.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19.
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951.
- Saccoliti, F.; Di Santo, R.; Costi, R. Recent Advancement in the Search of Innovative Antiprotozoal Agents Targeting Trypanothione Metabolism. ChemMedChem 2020, 1–17.
- Sarma, N.; Patouillard, E.; Cibulskis, R.E.; Arcand, J.L. The economic burden of Malaria: Revisiting the evidence. Am. J. Trop. Med. Hyg. 2019, 101, 1405–1415.
- World Health Organization. Malaria. Available online: (accessed on 15 December 2020).
- World Health Organization. Leishmaniasis. Available online: (accessed on 15 December 2020).
- World Health Organization. Human African Trypanosomiasis (Sleeping Sickness). Available online: (accessed on 15 December 2020).
- World Health Organization. Chagas Disease (American Trypanosomiasis). Available online: (accessed on 15 December 2020).
- Manner, C.K.; Graef, K.M.; Dent, J. WIPO Re:Search: Catalyzing public-private partnerships to accelerate tropical disease drug discovery and development. Trop. Med. Infect. Dis. 2019, 4, 53.
- Bernal, F.A.; Kaiser, M.; Wünsch, B.; Schmidt, T.J. Structure−Activity Relationships of Cinnamate Ester Analogues as Potent Antiprotozoal Agents. ChemMedChem 2020, 15, 68–78.
- Chen, Q.-h.; Liu, J.; Zhang, H.-f.; He, G.-q.; Fu, M.-l. The betulinic acid production from betulin through biotransformation by fungi. Enzym. Microb. Technol. 2009, 45, 175–180.
- Liu, J.; Fu, M.L.; Chen, Q.H. Biotransformation optimization of betulin into betulinic acid production catalysed by cultured Armillaria luteo-virens Sacc ZJUQH100-6 cells. J. Appl. Microbiol. 2011, 110, 90–97.
- An, T.; Zha, W.; Zi, J. Biotechnological production of betulinic acid and derivatives and their applications. Appl. Microbiol. Biotechnol. 2020, 104, 3339–3348.
- Stork, G.; Uyeo, S.; Wakamatsu, T.; Grieco, P.; Labovitz, J. The Total Synthesis of Lupeol. J. Am. Chem. Soc. 1971, 93, 4945–4947.
- Surendra, K.; Corey, E.J. A short enantioselective total synthesis of the fundamental pentacyclic triterpene lupeol. J. Am. Chem. Soc. 2009, 131, 13928–13929.
- Kim, D.S.H.L.; Chen, Z.; Van Nguyen, T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612.
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560.
- Csuk, R.; Schmuck, K.; Schäfer, R. A practical synthesis of betulinic acid. Tetrahedron Lett. 2006, 47, 8769–8770.
- Ruzicka, L.; Lamberton, A.H.; Christie, E.W. Zur Kenntnis der Triterpene. 41. Mitteilung. Oxydation des Betulin-mono-acetats mit Chromtrioxyd zu sauren Produkten. Helv. Chim. Acta 1938, 1706–1717.
- Baltina, L.A.; Flekhter, O.B.; Nigmatullina, L.R.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Tolstikov, G.A. Lupane triterpenes and derivatives with antiviral activity. Bioorganic Med. Chem. Lett. 2003, 13, 3549–3552.
- Pichette, A.; Liu, H.; Roy, C.; Tanguay, S.; Simard, F.; Lavoie, S. Selective oxidation of betulin for the preparation of betulinic acid, an antitumoral compound. Synth. Commun. 2004, 34, 3925–3937.
- Wickholm, N.; Alakurtti, S.; Yli-Kauhaluoma, J.; Koskimies, J. Method for preparation of betulinic acid 2013. WO2013038316A1, 21 March 2013.