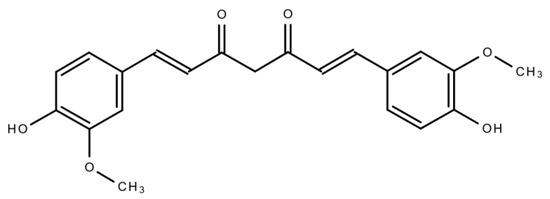
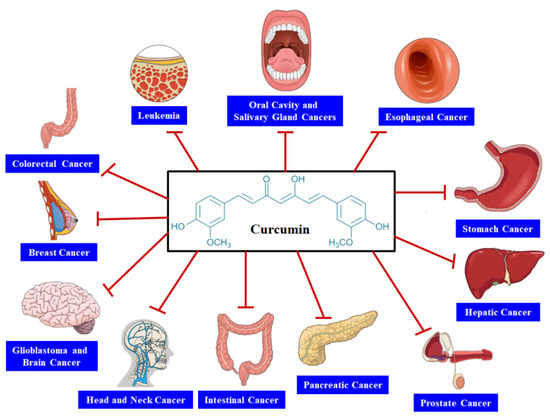
An imbalance between cell death and cell proliferation is regarded as one of the major causal factors of cancer [
17]. Uncontrolled cell proliferation is likely to occur if the cells skip death, which can lead to various types of cancers [
18]. The intrinsic and extrinsic pathways are responsible for generating apoptotic signals. It has been found that the intrinsic pathway plays a role via inducing the mitochondrial membrane to suppress the expressions of B-cell lymphoma-extra large and B-cell lymphoma 2 (Bcl-2) [
19]. CUR has the ability to disrupt the balance of mitochondrial membrane potential, which can result in increased Bcl-xL suppression [
20]. On the other hand, the extrinsic apoptotic pathway functions via inducing the tumor necrosis factor (TNF)-associated apoptosis and elevating the death receptors (DRs) on cells. In this pathway, CUR plays a role via upregulating DR4 and DR expression [
21,
22,
23]. It has been revealed by in vitro studies that CUR and its derivatives can excellently stimulate apoptosis in various cell lines through downregulating or suppressing intracellular transcription factors. These transcription factors include matrix metalloproteinase-9 (MMP-9), signal transducer and activator of transcription 3 (STAT3), cyclooxygenase II (COX-2), activator protein 1 (AP-1), nuclear factor-kappa B (NF-κB), and nitric oxide synthase [
24,
25]. CUR can also exert its anticancer effect via reducing lactate production and uptake of glucose in cancer cells through pyruvate kinase M2 (PKM2)downregulation. PKM2 suppression was found to be attained by inhibiting the mammalian target of rapamycin-hypoxia-inducible factor 1α (mTOR-HIF1α) [
26]. Multiple analyses have examined the capacity of CUR and derivatives of CUR to inhibit several types of cancers through interacting with various molecular targets ().
In CL-5 xenograft tumors, CUR can trigger apoptosis and caused downregulation of cyclin D1, c-Met, Akt, and epidermal growth factor receptor (EGFR) [
27]. Furthermore, CUR suppressed metastasis and lung cell invasion via upregulating the expression of HLJ1 in cancer cells [
28]. Along with the activity of CUR on nuclear factor-κB (NF-κB) and STAT3 signaling cascades, CUR also suppressed cell cycle arrest and cell proliferation and induced apoptosis by modulating other transcription factors including PPAR-α, Hif-1, Notch-1, β-catenin, p53, Erg-1, and AP-1 [
29]. It has been confirmed that CUR suppressed the phosphorylation of focal adhesion kinase (FAK) and increased the expression of multiple extracellular matrix (ECM) components, which further contribute to metastasis and invasion. In a concentration-dependent manner, CUR also increased cell adhesion via inducing various ECM components including fibronectin, laminin, collagen IX, collagen IV, collagen III, and collagen I. Collectively, these findings have indicated that CUR inhibits FAK action via suppression of its phosphorylation sites and triggers ECM components to improve cell adhesion, which can eventually prevent cell migration and detachment of cancer cells. It was reported that suppression of FAK expression resulted in elevated cell adhesion, which eventually playeda role in the anti-invasive activity of CUR [
30]. In colorectal cancer cells, CUR decreased the expression of CD24 in a dose-dependent manner. In addition, expression of E-cadherin was elevated by CUR and played a role as a suppressor of epithelial-mesenchymal transition. In colorectal cancer cells, CUR may exhibit its action against metastasis by downregulating CD24, FAK, and Sp-1, and upregulating the expression of E-cadherin [
30]. In a study, Zhou et al. [
30] assessed the activity of 11 CUR-associated compounds (comprising a benzyl piperidone moiety) in various cancer cell lines. Furthermore, they observed that some of these compounds decreased the level of the phospho-extracellular signal-regulated kinase (Erk)1/2 and phospho-Akt [
30]. It has been reported that autophagy and ER stress might have a significant contribution in the case of apoptosis, which is triggered via the CUR analogue B19 in hepatocellular carcinoma cells and the epithelial ovarian tumor cell line, and that suppression of autophagy may elevate CUR analogue-triggered apoptosis via stimulating severe ER stress. In ovarian cancer cell lines, this CUR analogue might also induce apoptosis, autophagy, and ER stress in vitro [
31,
32]. It was confirmed that autophagy may play role in programmed cell death type II and might effectively inhibit the growth of malignant glioma cells after treatment with CUR [
33].