Conjugated linoleic acids (CLA) are distinctive polyunsaturated fatty acids. They are present in food produced by ruminant animals and they are accumulated in seeds of certain plants. These naturally occurring substances have demonstrated to have anti-carcinogenic activity. Their potential effect to inhibit cancer has been shown in vivo and in vitro studies. In this study, we present the multiple effects of CLA isomers on cancer development such as anti-tumor efficiency, anti-mutagenic and anti-oxidant activity.
- conjugated linoleic acids (CLA)
- anti-cancer
- anti-tumor
- anti-mutagenic
- anti-oxidant
- proliferation
- apoptosis
1. Introduction
Fatty acids are important elements of the human body, having biological, structural and maintenance roles. There is great research of interest towards fatty acids and their potential health benefits [1]. Depending on the presence or absence of the double bonds, fatty acids are classified into two major classes: saturated and unsaturated. Saturated fatty acids contain only single bonds, whereas unsaturated fatty acids contain double or triple bonds. Unsaturated fatty acids that contain two or more double bonds are referred to as polyunsaturated fatty acids (PUFA). There are various types of PUFA, classified by their chemical structure. The first type includes fatty acids whose double bonds are separated by methylene group (-CH2-), all found with cis configuration. These types of naturally occurring fatty acids are known as essential fatty acids. Common non-conjugated PUFA are linoleic or ω−6 (9 cis, 12 cis-C18:2), found in nuts, seed and vegetable oils, and α-linolenic or ω−3 (9 cis, 12 cis, 15 cis-C18:3), found in seed, oil of plants, fish and seafood. In the second type, the double bonds have conjugated bonds and include fatty acids in multiple positional (9 cis, 11 trans-C18:2 and 10 trans, 12 cis-C18:2) isomers known as conjugated fatty acids (CFA). A group of CFA, containing 18 carbons and 2 conjugated bonds are known as conjugated linoleic acids (CLA), those having 18 carbons and 3 double bonds are referred to as conjugated linolenic acids (CLNA) and those having 20 carbons and 5 double bonds are conjugated eicosapentaenoic acids (CEPA). A well-studied example of conjugated fatty acids is CLA.
CLA are naturally occurring isomers of fatty acids found in ruminant animal food products [2]. There are 28 known CLA isomers identified with different position (ranging from Δ7, Δ9 to Δ12, Δ14) of cis or trans geometry. The most common biological isomers are the cis-9, trans-11(c9, t11), which accounts for more than 80% of CLA isomers in dairy products and cis-12, trans-10 (t10, c12), present in some ruminant fats [3]. The natural product of CLA is a mixture of two isomers: c9, t11 and t10, c12 available by partial hydrogenation of linoleic acid and/or isomerization of cis unsaturated fatty acids, using bacterial enzymes as catalysts (Figure 1) [4]. Many bacteria have been reported to convert free LA into CLA: Butyrivibrio fibrisolvens [5], Megasphaera elsdenii [6], Lactobacillus reuteri [7], L. acididophilus [8], L plantarum [9], and Bifidobacterium breve [10].
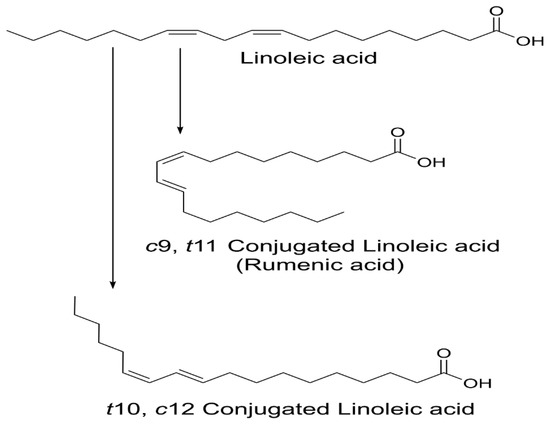
Figure 1. Structure of linoleic acid (LA), c9, t11 and t10, c12-CLA.
CLA is an essential, but minor component of fats that enters the human body primarily from the sources of dairy products and meat from ruminant animals [11]. The physiological properties of CLA have received considerable attention over the past few decades due to their documented health-promoting benefits and biological functions. The main health properties described of CLA include the reduction of carcinogenesis, diabetes, obesity and atherosclerosis in different animal and cell line studies [12]. Although CLA was firstly reported by Booth in 1935 in butterfat, one of the first health beneficial properties attributed to CLA was its anti-carcinogenic effects discovered in the late 70 s by Pariza et al. [13][14]. The CLA was described as an anti-carcinogen isolated from fried ground beef [14][15][16]. Since then, various studies (in vivo and in vitro) demonstrated that CLA could act by promoting the inhibitory effects on tumor cell growth [17]. However, the mechanisms of action through which CLA acts against cancer are not fully clear at present. Another important question is the dose and which isomer is necessary to bring the desired beneficial effects. In most of the studies, CLA mixture was demonstrated as a potential anti-cancer agent to regulate the tumor growth through different metabolic pathways and to alter lipid peroxidation, cell proliferation and apoptosis [18]. Some of the studies demonstrated the effective dose of a single isomer c9, t11-CLA, but not t10, c12-CLA [17][18][19]. Contrary to the initial studies, there have been few reports of CLA that did not show any inhibitory effects against cancer and even showed promotion on tumor progression [20].
2. Cellular Mechanisms by Which CLA May Inhibit Cancer
Overall, data from various cancer studies on animal, cell and human tumorigenesis with CLA provided abundant evidence that CLA can act as an anti-cancer agent. The examples of potential mechanisms of action to elucidate the role of CLA in modulating carcinogenesis on the stages of initiation, promotion, and progression are explained below.
2.1. Anti-Cancer Initiation
The anti-cancer initiation action of CLA can be attributed to the DNA damage leading to mutagenesis. The results from testing the chemoprotective effects on animal cell lines in the bladder (TSU-Pr1) showed that CLA decreased DNA synthesis and induced apoptosis in TSU-Pr1 cells. CLA was considered to act as an inhibitor of DNA synthesis [21]. Some studies originally believed that the anti-carcinogenic effects of CLA were due to its antioxidant activity. They reported the antioxidant activity of CLA by tracking the inhibition of CLA on the chelation of iron by β-hydroxy derivatives (Fenton reaction - catalysis of hydrogen peroxide) [14][15][22]. In a more recent study, Yu et al. demonstrated the protective effect of CLA against free scavenging radicals [23]. Another hypothesis suggested that the anti-carcinogenic activity of CLA was due to its antioxidant properties, promoting the in situ defense mechanism against free radical attacks on the cell membrane [24]. The supplementation of β-carotene in combination with CLA may be cytotoxic to human malignant melanoma and breast cancer cells in vitro [25]. CLA performed in vitro antioxidant activity by protecting the cells from peroxidation [23][25]. These results did not give any strong evidence and therefore it has not been conclusively established whether CLA works as an antioxidant. Further study is needed to resolve the relationship between the anti-cancer effects and antioxidant activity of CLA.
2.2. Anti-Cancer Promotion
The anti-promotional activity of CLA against cancer has been evaluated in several studies. The results suggested few potential mechanisms between CLA and animal/cell studies are likely to be involved: alternation of eicosanoid metabolism (COX-2, 5-LOX), induction of apoptosis (bcl-2), cell proliferation (c-myc), effects of PPAR. Belury et al. suggested that CLA affects the composition of the cell membrane, which, in turn alters eicosanoid synthesis [26]. A few studies also suggested that CLA acts as a preventive agent on eicosanoid metabolism which has a focus on tumor induction [27]. The reduction of eicosanoid was also monitored by the inhibition of the glycoprotein enzyme COX, responsible for the synthesis of prostaglandins and thromboxanes from arachidonic acid. Urquhart et al. investigated the role of CLA in the regulation of eicosanoid synthesis. They used selective inhibitor (indomethacin) for COX-1 and incubated it on human saphenous vein endothelial cells (HSVEC) with CLA (50:50, mixture of c9, t11 and t10, c12-CLA). The addition of CLA to HSVEC cells resulted in inhibition of eicosanoid production, suggesting that the cancer-preventive role of CLA in carcinogenesis was mediated through the inhibition of COX-1. The CLA mixture proved its beneficial anti-inflammatory effects that contributed to its anti-carcinogenic properties [27].
2.3. Anti-Proliferative
Previous studies in this report have documented the anti-proliferative properties of CLA. Tumorigenesis can be reduced by inhibiting cell growth or by increasing apoptosis. CLA has been demonstrated to stimulate the accumulation of proteins such as p27, p53 and p21, which have an additional role in tumor suppression [28][29][30]. Previously, CLA was presented to induce several tumors present in mammary, breast, colon and prostate tissues. The biochemical and molecular mechanisms to describe its anti-tumor effects have been suggested, but at the cellular level, exclusion of cancerous cells was manifested through a process of programmed cell death, called apoptosis [31][32][33][34]. Apoptosis depends on the activity of the Bcl-2 regulator proteins which are believed to work as an apoptosis “suppressor gene” [35]. In mitochondria, the release of cytochrome c to the cytosol is partially controlled by members of the Bcl-2 family. They inhibit the onset of apoptosis by blockage of the protein-conducting channels. They select and maintain the long-living cells in the G0 phase of the cell cycle [36]. Previously, we reported that Bcl-2 expression was decreased in various tumor lesions of animals fed with CLA [37][33]. It was showed that CLA inhibited the proliferation of colon and prostate cancer cells through the induction of apoptosis, attributing to its ability to down-regulate ErbB3 signaling and PI3/Akt pathway [37][38]. Furthermore, another study demonstrated that CLA targeted Bcl-2 by triggering apoptosis of p53 mutant mammary tumor cells [39]. As follows, the anti-proliferative effects of CLA appeared to be linked to the induction of apoptosis. In particular, we proposed a schematic model of the mechanism on CLA against carcinogenesis. It has been previously reported that CLA induced lipid metabolism and activated a group of nuclear transcription factors (PPARn). We predicted a model of peroxisome proliferation induced by CLA which resulted in apoptosis. The model was inspired by CLA inhibition in hepatic tumor cell lines (Figure 2). More details on the effects of each individual molecule can be found in the experimental studies [40][41][42][43].
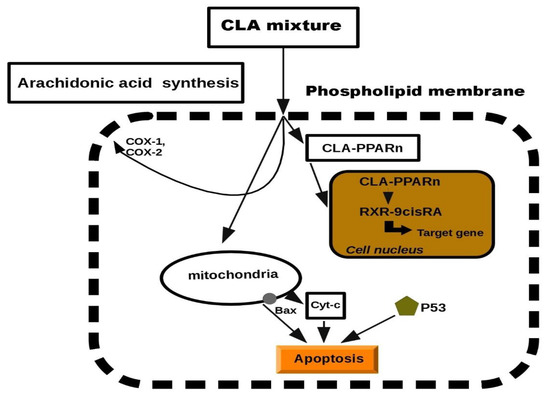
Figure 2. Schematic model of cell apoptosis induced by CLA.
This entry is adapted from the peer-reviewed paper 10.3390/pr9030454
References
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172.
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532.
- Albers, R.; van der Wielen, R.P.; Brink, E.J.; Hendriks, H.F.; Dorovska-Taran, V.N.; Mohede, I.C. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur. J. Clin. Nutr. 2003, 57, 595–603.
- Salsinha, A.S.; Pimentel, L.L.; Fontes, A.L.; Gomes, A.M.; Rodríguez-Alcalá, L.M. Microbial Production of Conjugated Linoleic Acid and Conjugated Linolenic Acid Relies on a Multienzymatic System. Microbiol. Mol. Biol. Rev. 2018, 82, e00019-18.
- Kepler, C.R.; Tucker, W.P.; Tove, S.B. Biohydrogenation of Unsaturated Fatty Acids: V. Stereospecificity of proton addition and mechanism of action of linoleic acid Δ12-cis, Δ11-trans-isomerase from Butyrivibrio fibrisolvens. J. Biol. Chem. 1971, 246, 2765–2771.
- Gorissen, L.; Leroy, F.; De Vuyst, L.; De Smet, S.; Raes, K. Bacterial production of conjugated linoleic and linolenic Acid in foods: A technological challenge. Crit. Rev. Food Sci. Nutr. 2015, 55, 1561–1574.
- Lee, S.O.; Kim, C.S.; Cho, S.K.; Choi, H.J.; Ji, G.E.; Oh, D.-K. Bioconversion of linoleic acid into conjugated linoleic acid during fermentation and by washed cells of Lactobacillus reuteri. Biotechnol. Lett. 2003, 25, 935–938.
- Lin, M.-Y.; Yen, C.-L. Inhibition of Lipid Peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J. Agric. Food Chem. 1999, 47, 3661–3664.
- Dahiya, D.K.; Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J. Food Sci. Technol. 2017, 54, 792–801.
- Chung, S.H.; Kim, I.H.; Park, H.G.; Kang, H.S.; Yoon, C.S.; Jeong, H.Y.; Choi, N.J.; Kwon, E.G.; Kim, Y.J. Synthesis of Conjugated Linoleic Acid by Human-Derived Bifidobacterium breve LMC 017: Utilization as a Functional Starter Culture for Milk Fermentation. J. Agric. Food Chem. 2008, 56, 3311–3316.
- Dhiman, T.R.; Nam, S.H.; Ure, A.L. Factors affecting conjugated linoleic acid content in milk and meat. Crit. Rev. Food Sci. Nutr. 2005, 45, 463–482.
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, W. Review of the roles of conjugated linoleic acid in health and disease. J. Funct. Foods 2015, 15, 314–325.
- Booth, R.G.; Kon, S.A. A study of seasonal variation in butter fat: A seasonal spectroscopic variation in the fatty acid fraction. Biochem. J. 1935, 29, 133–137.
- Pariza, M.W.; Ashoor, S.H.; Chu, F.S. Mutagens in heat-processed meat, bakery and cereal products. Food Cosmet Toxicol. 1979, 17, 429–430.
- Ha, Y.L.; Grimm, N.K.; Pariza, M.W. Anticarcinogens from fried ground beef: Heat-altered derivatives of linoleic acid. Carcinogenesis 1987, 8, 1881–1887.
- Tanaka, K. Occurrence of conjugated linoleic acid in ruminant products and its physiological functions. Anim. Sci. J. 2005, 76, 291–303.
- Kelley, N.S.; Hubbard, N.E.; Erickson, K.L. Conjugated linoleic acid isomers and cancer. J. Nutr. 2007, 137, 2599–2607.
- Lee, K.W.; Lee, H.J.; Cho, H.Y.; Kim, Y.J. Role of the conjugated linoleic acid in the prevention of cancer. Crit. Rev. Food Sci. Nutr. 2005, 45, 135–144.
- Khanal, R. Potential health benefits of conjugated linoleic acid (CLA): A Review. Anim. Biosci. 2004, 17, 1315–1328.
- Den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients. 2019, 11, 370.
- Oh, Y.S.; Lee, H.S.; Cho, H.J.; Lee, S.G.; Jung, K.C.; Park, J.H. Conjugated linoleic acid inhibits DNA synthesis and induces apoptosis in TSU-Pr1 human bladder cancer cells. Anticancer Res. 2003, 23, 4765–4772.
- Ha, Y.L.; Storkson, J.; Pariza, M.W. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990, 50, 1097–1101.
- Yu, L. Free Radical Scavenging Properties of Conjugated Linoleic Acids. J. Agric. Food Chem. 2001, 49, 3452–3456.
- Shultz, T.D.; Chew, B.P.; Seaman, W.R.; Luedecke, L.O. Inhibitory effect of conjugated dienoic derivatives of linoleic acid and beta-carotene on the in vitro growth of human cancer cells. Cancer Lett. 1992, 63, 125–133.
- Lalithadevi, B.; Muthiah, N.S.; Murty, K.S.N. Antioxidant activity of conjugated linoleic acid. Asian J. Pharm. Clin. Res. 2018, 11, 169.
- Belury, M.A.; Kempa-Steczko, A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 1997, 32, 199–204.
- Urquhart, P.; Parkin, S.M.; Rogers, J.S.; Bosley, J.A.; Nicolaou, A. The effect of conjugated linoleic acid on arachidonic acid metabolism and eicosanoid production in human saphenous vein endothelial cells. Biochim. Biophys Acta 2002, 1580, 150–160.
- Kim, K.-H.; Park, H.-S. Dietary supplementation of conjugated linoleic acid reduces colon tumor incidence in DMH-treated rats by increasing apoptosis with modulation of biomarkers. Nutrition 2003, 19, 772–777.
- Kemp, M.Q.; Jeffy, B.D.; Romagnolo, D.F. Conjugated linoleic acid inhibits cell proliferation through a p53-dependent mechanism: Effects on the expression of G1-restriction points in breast and colon cancer cells. J. Nutr. 2003, 133, 3670–3677.
- Cho, H.J.; Kim, E.J.; Lim, S.S.; Kim, M.K.; Sung, M.K.; Kim, J.S.; Park, J.H. Trans-10,cis-12, not cis-9,trans-11, conjugated linoleic acid inhibits G1-S progression in HT-29 human colon cancer cells. J. Nutr. 2006, 136, 893–898.
- Park, H.S.; Ryu, J.H.; Ha, Y.L.; Park, J.H.Y. Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1,2-dimethylhydrazine-treated rats: A possible mechanism of the anticarcinogenic effect by CLA. Br. J. Nutr. 2001, 86, 549–555.
- Miglietta, A.; Bozzo, F.; Bocca, C.; Gabriel, L.; Trombetta, A.; Belotti, S.; Canuto, R.A. Conjugated linoleic acid induces apoptosis in MDA-MB-231 breast cancer cells through ERK/MAPK signalling and mitochondrial pathway. Cancer Lett. 2006, 234, 149–157.
- Ip, C.; Ip, M.M.; Loftus, T.; Shoemaker, S.; Shea-Eaton, W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol. Biomark. Prev. 2000, 9, 689–696.
- Wang, L.S.; Huang, Y.W.; Liu, S.; Yan, P.; Lin, Y.C. Conjugated linoleic acid induces apoptosis through estrogen receptor alpha in human breast tissue. BMC Cancer 2008, 8, 208.
- Bostwick, D.G.; Meiers, I. Prostate. In Modern Surgical Pathology, 2nd ed.; Weidner, N., Cote, R., Suster, S., Weiss, L., Eds.; Saunders: Collingwood, ON, Canada, 2009; Volume 2, pp. 1121–1180.
- Borner, C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003, 39, 615–647.
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H.Y. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G996–G1005.
- Cho, H.J.; Kim, W.K.; Jung, J.I.; Kim, E.J.; Lim, S.S.; Kwon, D.Y.; Park, J.H. Trans-10,cis-12, not cis-9,trans-11, conjugated linoleic acid decreases ErbB3 expression in HT-29 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5142–5150.
- Ou, L.; Ip, C.; Lisafeld, B.; Ip, M.M. Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss. Biochem. Biophys. Res. Commun. 2007, 356, 1044–1049.
- Yamasaki, M.; Chujo, H.; Koga, Y.; Oishi, A.; Rikimaru, T.; Shimada, M.; Sugimachi, K.; Tachibana, H.; Yamada, K. Potent cytotoxic effect of the trans10, cis12 isomer of conjugated linoleic acid on rat hepatoma dRLh-84 cells. Cancer Lett. 2002, 188, 171–180.
- Yamasaki, M.; Miyamoto, Y.; Chujo, H.; Nishiyama, K.; Tachibana, H.; Yamada, K. Trans10, cis12-conjugated linoleic acid induces mitochondria-related apoptosis and lysosomal destabilization in rat hepatoma cells. Biochim. Biophys. Acta (Bba) Mol. Cell Biol. Lipids 2005, 1735, 176–184.
- Muzio, G.; Maggiora, M.; Oraldi, M.; Trombetta, A.; Canuto, R.A. PPARα and PP2A are involved in the proapoptotic effect of conjugated linoleic acid on human hepatoma cell line SK-HEP-1. Int. J. Cancer 2007, 121, 2395–2401.
- Yamasaki, M.; Nagatomo, T.; Matsuyama, T.; Ikeho, Y.; Kato, E.; Nishiyama, K.; Sakakibara, Y.; Suiko, M.; Nishiyama, K. Conjugated linoleic acids inhibit hypoxia inducible factor-1α stabilization under hypoxic condition in human hepatocellular carcinoma cells. J. Oleo Sci. 2012, 61, 491–496.
