Mesenchymal stromal/stem cells (MSCs) are multipotent adult stem cells that support homeostasis during tissue regeneration.
- MSCs
1. Introduction
Mesenchymal stromal/stem cells (MSCs) belong to the pool of adult stem cells that, in a specific microenvironment termed the “stem cells niche”, support tissue regeneration in both physiologic and pathologic conditions contributing to tissue homeostasis[1][2][3]. These cells can increase their own compound[4] and replace individual components of the microenvironment by differentiating or attracting supporting cells to a niche[1][2][3].
2. Role
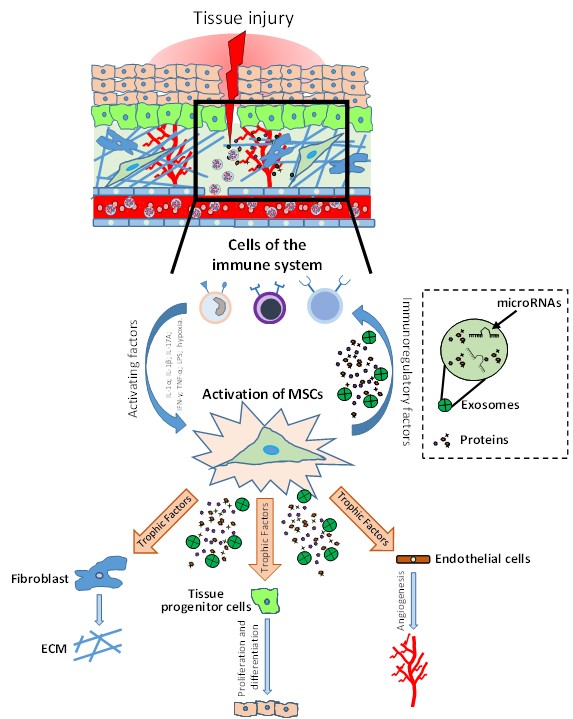
It has been shown that in tissues such as intestine and skin, MSCs support a high cellular turnover[5][6]. In contrast, in other tissues such as skeletal muscle, MSCs are considered adult stem cells that support regeneration after injury, even if they marginally contribute to myofiber renewal during physiologic turnover[7]. Indeed, MSC-like cells with adipogenic phenotype resident in the muscles are quiescent in intact tissue, but get activated in injured ones, providing a momentary source of key factors that induce proliferation of myogenic progenitor cells. Thus, these MSCs that normally have adipogenic potential, when injury-activated, can stimulate the differentiation of the myogenic progenitor’s cells supporting tissue repair[3][8]. A similar phenomenon has been shown in skin tissue, where MSC-like adipose precursor cells within the skin appear to be crucial for epithelial cell regulation[9]. Therefore, MSCs can be considered as key regulatory components in the regenerating stem cell niche (Figure 1).
Figure 1. Role of mesenchymal stromal/stem cells (MSCs) in tissue injury and repair. After injury, the damaged tissue activates MSCs through different inflammatory signals (IL-1β, IFN-γ, TNF-α, LPS; hypoxia). MSC activation leads to coordination of the microenvironment by both the production of immunomodulatory factors (to modulate the progression of inflammation) and the production of growth factors which subsequently stimulate endothelial cells, fibroblasts cells and tissue progenitor cells. The physiological and orderly action of these factors allows tissue repair through angiogenesis, remodeling of the extracellular matrix (ECM) and functional tissue restoration through tissue progenitor cells differentiation.
These findings provide a robust rationale to investigate the role of MSCs as a therapeutic product to support tissue injury responses in different diseases. MSCs are multipotent cells with easy accessibility, few ethics-related issues, and higher adaptability to in vitro cultures for expansion. Unlike pluripotent stem cells, multipotent MSCs have a limited self-renewal capacity[10]. In light of this, in recent years the “stem” cell definition has been changed to “stromal” in order to give a more appropriate connotation to describe MSCs. Furthermore, these cells are immuno-privileged due to their low expression of CD40, CD80, CD86 and major histocompatibility complex I (MHC I), as well as the lack of MHC II expression[11][12][13][14]. These features make these cells a very useful tool for cell therapy in the field of regenerative medicine.
MSCs are found in several tissues, including bone marrow (BM)[15], adipose tissue (AT)[16], umbilical cord (UC)[17], dental pulp[18] and placenta[19], where these cells are surrounded by different cell types such as immune cells, epithelial cells, endothelial cells and stromal cells, and can exhibit immunomodulatory[20][21], angiogenic[22][23] and anti-oxidative properties[24]. Over the past decade the therapeutic action of MSCs has been investigated in several clinical trials for the treatment of many disorders including cardiovascular, neurodegenerative, immune, lung, liver, kidney and orthopedics diseases (clinicaltrials.gov). In these cases, MSCs have been shown to have moderate or poor efficacy, and the results from different clinical trials are controversial[25][26][27][28][29], indicating an urgent need to optimize the therapeutic use of MSCs or to enhance MSC potency. This inconsistent evidence is potentially related both to intrinsic differences in the use of cell-based products and to the lack of standardized methods for MSC production that affects their potency. MSC effects depend both on tissue source[30][31] and on how they are produced and administered. Indeed, it has been shown that the composition of MSCs secretome can be modulated by preconditioning of MSCs with hypoxia and cytokines treatments, as well as the growing of MSCs under specific culture systems, including three-dimensional (3D) culture conditions[32][33][34][35]. In response to MSC “priming”, the production of factors is switched towards an anti-inflammatory and pro-trophic phenotype that results in a homeostatic regulation of tissue regeneration/repair[36][37]. Currently, it is often stated that the efficacy of MSCs therapies is probably not related to cell engraftment and replacement but is linked to the production of crucial paracrine factors, such as cytokines, growth factors, and exosomes (EXOs), that regulate the cell niche for their regeneration. Indeed, in response to specific stimuli, MSCs are activated and can secrete a plethora of regulating factors that affect tissue injury responses in a transitory and paracrine manner to orchestrate the repairing tissue processes[20][38][39][40][41][42][43][44]. In a different model of injury it has been shown that MSCs, mainly triggered by inflammation processes, induce tissue regeneration/repair by cell niche empowerment/regulation[45][46][47]. In these cases, in an inflammatory-injured tissue, MSC effects were mediated by paracrine mechanisms that lead to regulation of fibrosis, immunomodulation, stimulation of angiogenesis and stimulation of resident cells to coordinate both tissue regeneration and function recovery[37][48][49][50][51][52]. Therefore, due to the regenerative potential and trophic properties of specific MSC-derived products, such as the conditioned medium (CM) and EXOs, these products have emerged as possible therapeutic tools with numerous applications and are consequently being extensively evaluated for medical use[53][54][55]. In addition, the clinical application of MSC-derived products must be considered for their advantages as opposed both to the lack of safety in the long-term use of MSCs and the risks related to transmission of infection diseases, such as some viruses found in the transplanted allogenic cells.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020763
References
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453.
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334.
- Wosczyna, M.N.; Konishi, C.T.; Perez Carbajal, E.E.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035.e5.
- Tomer Itkin; Aya Ludin; Ben Gradus; Shiri Gur-Cohen; Alexander Kalinkovich; Amir Schajnovitz; Yossi Ovadya; Orit Kollet; Jonathan Canaani; Elias Shezen; et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood 2012, 120, 1843-1855, 10.1182/blood-2011-11-394692.
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33.
- Hsu, Y.C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856.
- Andrew S. Brack; Thomas A. Rando; Tissue-Specific Stem Cells: Lessons from the Skeletal Muscle Satellite Cell. Cell Stem Cell 2012, 10, 504-514, 10.1016/j.stem.2012.04.001.
- Aaron W. B. Joe; Lin Yi; Anuradha Natarajan; Fabien Le Grand; Leslie So; Joy Wang; Michael A. Rudnicki; Fabio M. V. Rossi; Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature 2010, 12, 153-163, 10.1038/ncb2015.
- Eric Festa; Jackie Fretz; Ryan Berry; Barbara Schmidt; Matthew Rodeheffer; Mark Horowitz; Valerie Horsley; Adipocyte Lineage Cells Contribute to the Skin Stem Cell Niche to Drive Hair Cycling. Cell 2011, 146, 761-771, 10.1016/j.cell.2011.07.019.
- Qingguo Zhao; Carl A. Gregory; Ryang Hwa Lee; Roxanne L. Reger; Lizheng Qin; Bo Hai; Min Sung Park; Nara Yoon; Bret Clough; Eoin McNeill; et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proceedings of the National Academy of Sciences 2014, 112, 530-535, 10.1073/pnas.1423008112.
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39.
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896.
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577.
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191.
- Sebastian G. Walter; Thomas M. Randau; Cäcilia Hilgers; El-Mustapha Haddouti; Werner Masson; Sascha Gravius; Christof Burger; Dieter C. Wirtz; Frank A. Schildberg; Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. International Journal of Molecular Sciences 2020, 21, 4382, 10.3390/ijms21124382.
- Blaž Burja; Ariana Barlič; Andreja Erman; Katjuša Mrak-Poljšak; Matija Tomšič; Snezna Sodin-Semrl; Katja Lakota; Human mesenchymal stromal cells from different tissues exhibit unique responses to different inflammatory stimuli. Current Research in Translational Medicine 2020, 68, 217-224, 10.1016/j.retram.2020.05.006.
- Lenka Tesarova; Klara Jaresova; Pavel Simara; Irena Koutna; Umbilical Cord-Derived Mesenchymal Stem Cells are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. International Journal of Molecular Sciences 2020, 21, 5366, 10.3390/ijms21155366.
- S. Gronthos; M. Mankani; J. Brahim; P. Gehron Robey; S. Shi; Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proceedings of the National Academy of Sciences 2000, 97, 13625-13630, 10.1073/pnas.240309797.
- Ornella Parolini; Francesco Alviano; Gian Paolo Bagnara; Grozdana Bilic; Hans-Jörg Bühring; Marco Evangelista; Simone Hennerbichler; Bing Liu; Marta Magatti; Ning Mao; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. STEM CELLS 2008, 26, 300-311, 10.1634/stemcells.2007-0594.
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-gamma Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54.
- Poggi, A.; Zocchi, M.R. Immunomodulatory Properties of Mesenchymal Stromal Cells: Still Unresolved “Yin and Yang”. Curr. Stem Cell Res. Ther. 2019, 14, 344–350.
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. CMLS 2020, 77, 2771–2794.
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016, 1314709.
- Rhian Stavely; Kulmira Nurgali; The emerging antioxidant paradigm of mesenchymal stem cell therapy. STEM CELLS Translational Medicine 2020, 9, 985-1006, 10.1002/sctm.19-0446.
- Fricova, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020, 5, 20.
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536.
- Malliaras, K.; Kreke, M.; Marban, E. The stuttering progress of cell therapy for heart disease. Clin. Pharmacol. Ther. 2011, 90, 532–541.
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848.
- Tyndall, A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 280–284.
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359.
- Schmelzer, E.; Miceli, V.; Chinnici, C.M.; Bertani, A.; Gerlach, J.C. Effects of Mesenchymal Stem Cell Coculture on Human Lung Small Airway Epithelial Cells. BioMed Res. Int. 2020, 2020, 9847579.
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 1276–1292.
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837.
- Miceli, V.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. Biochem. Biophys. Res. Commun. 2020, 522, 171–176.
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279.
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79.
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016.
- Neirinckx, V.; Coste, C.; Rogister, B.; Wislet-Gendebien, S. Concise review: Adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: A state of play. Stem Cells Transl. Med. 2013, 2, 284–296.
- Nuzzi, R.; Caselgrandi, P.; Vercelli, A. Effect of Mesenchymal Stem Cell-Derived Exosomes on Retinal Injury: A Review of Current Findings. Stem Cells Int. 2020, 2020, 8883616.
- Qian, X.; An, N.; Ren, Y.; Yang, C.; Zhang, X.; Li, L. Immunosuppressive Effects of Mesenchymal Stem Cells-derived Exosomes. Stem Cell Rev. Rep. 2020.
- Sinclair, K.; Yerkovich, S.T.; Chambers, D.C. Mesenchymal stem cells and the lung. Respirology 2013, 18, 397–411.
- Sun, H.; Pratt, R.E.; Hodgkinson, C.P.; Dzau, V.J. Sequential paracrine mechanisms are necessary for the therapeutic benefits of stem cell therapy. Am. J. Physiol. Cell Physiol. 2020, 319, C1141–C1150.
- Wang, M.; Yan, L.; Li, Q.; Yang, Y.; Turrentine, M.; March, K.; Wang, I.W. Mesenchymal stem cell secretions improve donor heart function following ex vivo cold storage. J. Thorac. Cardiovasc. Surg. 2020.
- Zhu, X.Y.; Lerman, A.; Lerman, L.O. Concise review: Mesenchymal stem cell treatment for ischemic kidney disease. Stem Cells 2013, 31, 1731–1736.
- Fayyad-Kazan, M.; Fayyad-Kazan, H.; Lagneaux, L.; Najar, M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy 2016, 8, 839–842.
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784.
- Xi, J.; Yan, X.; Zhou, J.; Yue, W.; Pei, X. Mesenchymal stem cells in tissue repairing and regeneration: Progress and future. Burn. Trauma 2013, 1, 13–20.
- Butler, J.; Epstein, S.E.; Greene, S.J.; Quyyumi, A.A.; Sikora, S.; Kim, R.J.; Anderson, A.S.; Wilcox, J.E.; Tankovich, N.I.; Lipinski, M.J.; et al. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ. Res. 2017, 120, 332–340.
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002.
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537.
- Sagaradze, G.; Basalova, N.; Kirpatovsky, V.; Ohobotov, D.; Nimiritsky, P.; Grigorieva, O.; Popov, V.; Kamalov, A.; Tkachuk, V.; Efimenko, A. A magic kick for regeneration: Role of mesenchymal stromal cell secretome in spermatogonial stem cell niche recovery. Stem Cell Res. Ther. 2019, 10, 342.
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209.
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313.
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630.