Chronic kidney disease (CKD) is estimated to affect more than 10% of the global population and represents an increasing health and economic burden for the society. Cardiovascular disease (CVD) is the most important complication of CKD and the primary cause of death in these patients. Chronic kidney disease-mineral and bone disorder (CKD-MBD), which is a common complication of CKD patients, involves changes in mineral ion homeostasis, bone quality and turnover, cardiovascular and soft tissue calcifications, highly contributing for cardiovascular outcomes. Vascular calcification (VC) is one of the strongest predictors of cardiovascular risk in chronic kidney disease (CKD) patients which is associated with significant morbidity and mortality. New diagnostic/prognostic tools are required for early detection of VC allowing interventional strategies. Gla-rich protein (GRP) is a cardiovascular calcification inhibitor, whose clinical utility still remained unknown. The present clinical study including a cohort of 80 diabetic patients with mild to moderate CKD (stages 2–4) explored, for the first time, correlations between levels of GRP in serum with CKD developmental stage, mineral metabolism markers, VC and pulse pressure (PP). The results shown an association between GRP, renal dysfunction and CKD-MBD. The relationship between low levels of GRP and vascular calcifications suggests a potential clinical utility for GRP as an early marker of vascular damage in CKD.
- cardiovascular calcification
- Gla-rich protein
- chronic kidney disease
- vascular calcification inhibitors
- cardiovascular risk assessment
- cardiovascular disease
1. Introduction
Epidemiologically, CKD, diabetes mellitus and atherosclerosis are the clinical conditions that most contribute towards development of vascular and valves calcification[1][2]. Although the relevance of vascular calcification (VC) assessment is recognized in clinical practice, most of the available quantitative methods have many shortcomings[3]. Therefore, the development of biomarkers for early detection of VC are crucial for the prevention of CVD outcomes in CKD patients, allowing preventive measures to reduce the development and progression of VC, left ventricular hypertrophy and arterial stiffness. VC is a highly-controlled multifactorial process that requires constant inhibition[4]. High prevalence of VC in CKD patients is suggested to result from several interconnected processes involving dysregulation of endogenous calcification inhibitors, abnormal mineral metabolism and inflammation[5]. Biochemical targets known to be involved in these pathological processes have been explored for their potential use as biomarkers for VC and cardiovascular risk assessment[4]. Gla-rich protein (GRP), also known as upper zone of growth plate and cartilage matrix associated protein (UCMA)[6], is a circulating vitamin K-dependent protein (VKDP) with a dual capacity to function as an inhibitor of pathological calcification and anti-inflammatory agent, in the articular and cardiovascular systems[7][8][9][10]. Although GRP has been suggested as a potential marker for VC, its clinical utility has never been shown.
In this first GRP clinical study, a clear association between circulating levels of GRP and the progression of CKD pathology and vascular calcification was shown. This study included all eligible type 2 diabetic patients with CKD stages 2-4 (n=80) followed in outpatient nephrology consultation from 2010 to 2017, where correlations between levels of GRP in serum with mineral metabolism and inflammation markers, CKD developmental stage, VC and pulse pressure (PP), were explored. Vascular calcification score (VCS) was evaluated using the plain x-ray of the hands and pelvis (Adragão score), and increased cardiovascular risk was considered for VCS≥3. Measurements of GRP in serum were performed using a recently developed sandwich ELISA (GenoGla Diagnostics)[9].
2.1 Decreased GRP serum levels is associated with increased VC promoters and decreased VC inhibitors
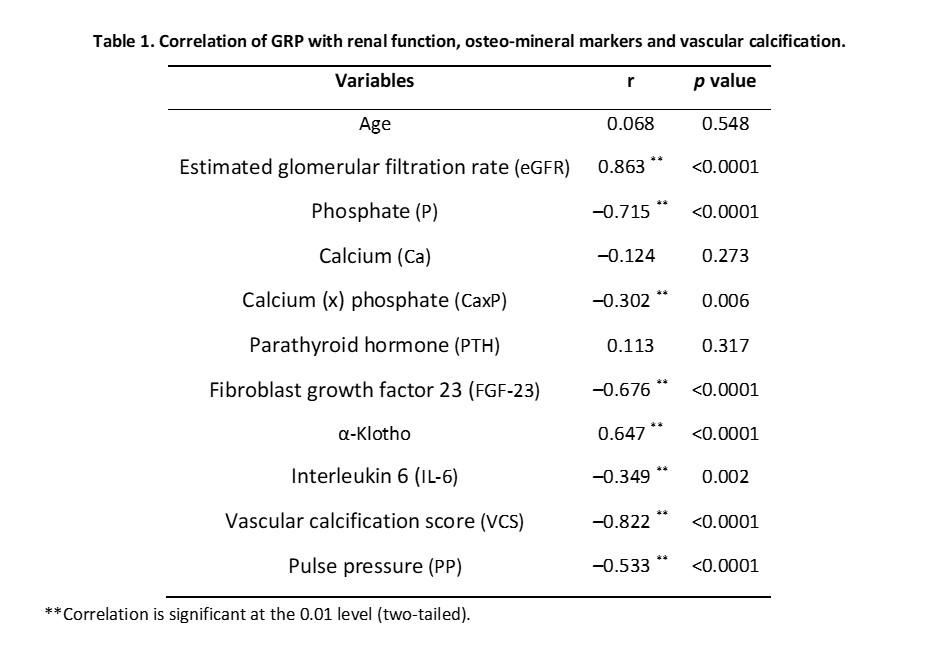
Spearman’s correlation analysis revealed statistically significant strong positive correlations between GRP serum levels and eGFR and α-Klotho; strong negative correlations with phosphate (P), FGF23, VCS and PP; and moderate negative correlations with CaxP and the inflammation marker IL-6 (Table 1). These results demonstrate that a reduction in GRP levels associate with an increase in levels of the VC promoters P, FGF-23 and CaxP, and a decrease in the VC inhibitor α-Klotho, clearly showing a correlation between GRP and the dysregulation of phosphate metabolism characteristic of CKD-MBD.
2.2 GRP serum levels progressively decrease with the deterioration of renal function
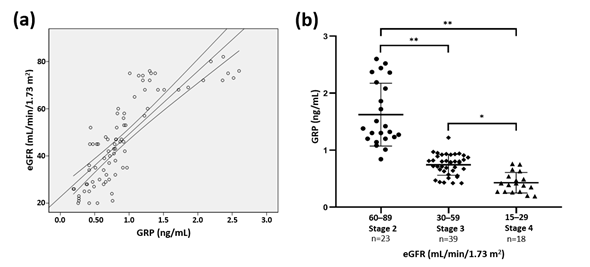
Simple linear regression analysis demonstrated a positive association between GRP serum levels and eGFR (Figure 1a), which remained significant after adjustments for age and gender (r = 0.823, p < 0.0001). Furthermore, GRP levels were shown to significantly decrease from stage 2 to stage 4 CKD (Figure 1b). These results shown that a decrease in eGFR is accompanied by a decrease in GRP levels from CKD stage 2 to stage 4, indicating GRP as a possible early marker associated with renal dysfunction. Although the current study does not allow us to infer on the causality of this relationship, it has been demonstrated that the prevalence of VC increases as eGFR declines while low eGFR is associated with cardiovascular morbidity and mortality[11][12][13]. Moreover, VC is a suggested link between low eGFR and worse cardiovascular outcome[14][15]. It is presently unclear whether the relationship between GRP and eGFR might be beyond VC, eventually involving kidney disease physiopathology.
Figure 1. Association between serum Gla-rich protein (GRP) levels and kidney function. (a) The simple linear regression was used to assess the relationship between estimated glomerular filtration rate (eGFR) and serum GRP levels (β = 0.821; p < 0.0001). (b) Serum GRP levels divided by chronic kidney disease (CKD) stage. ANOVA test and a post hoc analysis with Scheffe test was used to analyse differences among the 3 groups (* p = 0.001, ** p < 0.0001).
2.3 GRP is associated with the vascular calcification score (VCS) and pulse pressure (PP) in multivariate logistic regression models
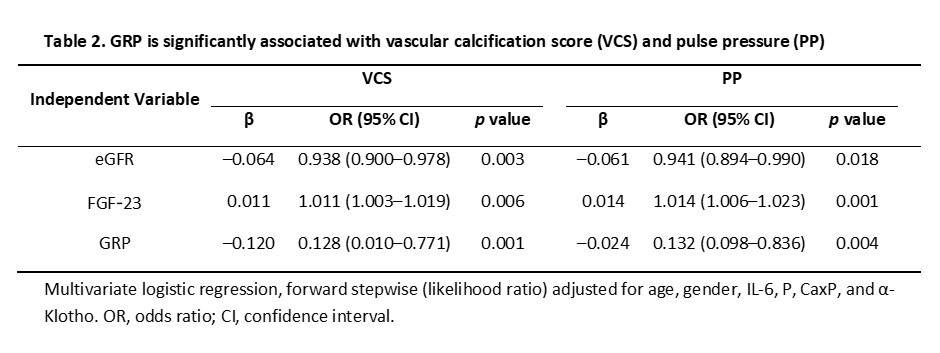
In univariate logistic regression analysis, eGFR, P, CaxP, FGF-23, α-Klotho, IL-6 and GRP were associated with the VCS and PP. From those, only eGFR, GRP and FGF-23 were found significantly associated with both the VCS and PP in multivariate logistic regression models (Table 2).
2.4 Discussion and Conclusion
This is the first clinical study showing the association of circulating levels of GRP with CKD progression and vascular calcification. It was shown that, in adult diabetic CKD patients, serum GRP levels progressively decrease from stage 2 to stage 4 CKD, correlating with markers of mineral metabolism, vascular calcification and pulse pressure. Moreover, low levels of GRP were strongly associated with vascular calcification and pulse pressure, providing support to the hypothesis of being considered as a novel cardiovascular risk factor in this population.
The relationship between bone mineral disorders and VC is well established and a major concern on the management of cardiovascular risk in the CKD population. In this complex interplay, the contribution of phosphate metabolism for VC and cardiovascular outcomes in CKD settings has been widely demonstrated[4][3]. Increased levels of serum P, FGF-23 and CaxP, and lower levels of α-Klotho, have all been associated with cardiovascular outcomes in the CKD population[4][16][17]. The association of GRP with CKD-MBD and VC are consistent with reported data regarding GRP functionality. GRP has been shown to be involved in VC inhibition at multiple levels, through the inhibition of VSMCs osteochondrogenic differentiation or the direct inhibition of mineral formation, maturation and growth, both in circulation and at the vascular tissue[6][9][18].
Limitations of our study include the small sample size, the fact that serum GRP levels were measured at a single point, and the absence of reference intervals for GRP levels in a healthy population. Also, this study included subjects with mild to moderate CKD followed in outpatient diabetic nephropathy clinic and may not be representative of kidney disease of other etiology. The main strength of this study clearly resides in the novelty concerning the clinical utility of GRP. The presented data show that decreased levels of GRP in circulation parallels the progression of CKD and increased vascular calcification, suggesting a future potential use of GRP as an early marker of vascular damage in CKD patients.
Patents:
The tools and methods described in this manuscript are included in a PCT patent application PCT/PT2009000046.
Conflicts of Interest:
Dina C. Simes and Carla Viegas are cofounders of GenoGla Diagnostics. The authors declare that there are no conflicts of interest regarding the publication of this paper. The tools and methods described in this manuscript are included in a PCT patent application PCT/PT2009000046, which is owned by University of Algarve and the Centre of Marine Sciences (CCMAR) and the exclusive rights are licensed to GenoGla Diagnostics.
Funding:
This research was funded by the Portuguese Society of Nephrology (SPN) through projects funding 2017 and 2019, by the Portuguese national funds from FCT—Foundation for Science and Technology through the transitional provision DL57/2016/CP1361/CT0006 and project UIDB/04326/2020.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9030635
References
- Masahide Mizobuchi; Dwight Towler; Eduardo Slatopolsky; Vascular Calcification: The Killer of Patients with Chronic Kidney Disease. Journal of the American Society of Nephrology 2009, 20, 1453-1464, 10.1681/asn.2008070692.
- Giuseppe Vezzoli; Alessandro Rubinacci; Monica Lazzaroni; Laura Soldati; It’s time for a practical method quantifying vascular calcification. Journal of Translational Medicine 2014, 12, 172-172, 10.1186/1479-5876-12-172.
- Jane A. Leopold; Vascular calcification: Mechanisms of vascular smooth muscle cell calcification.. Trends in Cardiovascular Medicine 2014, 25, 267-74, 10.1016/j.tcm.2014.10.021.
- Carla Viegas; Nuna Araújo; Catarina Marreiros; Dina C. Simes; The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): challenging old concepts with new facts.. Aging 2019, 11, 4274-4299, 10.18632/aging.102046.
- Cordula Surmann-Schmitt; Uwe Dietz; Trayana Kireva; Nadia Adam; Jung Park; Andreas Tagariello; Patrik Onnerfjord; Dick Heinegård; Ursula Schlötzer-Schrehardt; Rainer Deutzmann; et al. Ucma, a Novel Secreted Cartilage-specific Protein with Implications in Osteogenesis. Journal of Biological Chemistry 2007, 283, 7082-7093, 10.1074/jbc.m702792200.
- Carla S.B. Viegas; Marta Rafael; José L. Enriquez; Alexandra Teixeira; Rui Vitorino; Inês Matias Luís; Rúben M. Costa; Sofia Santos; Sofia Cavaco; José Neves; et al. Gla-Rich Protein Acts as a Calcification Inhibitor in the Human Cardiovascular System. Arteriosclerosis, Thrombosis, and Vascular Biology 2014, 35, 399-408, 10.1161/atvbaha.114.304823.
- Sofia Cavaco; Carla S.B. Viegas; Marta Rafael; Acácio Ramos; Joana Magalhães; Francisco J. Blanco; C. Vermeer; Dina C. Simes; Francisco J Blanco; Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cellular and Molecular Life Sciences 2015, 73, 1051-1065, 10.1007/s00018-015-2033-9.
- Carla S.B. Viegas; Rúben M. Costa; Lúcia Santos; Paula A. Videira; Zélia Silva; Nuna Araújo; Anjos Macedo; António Pedro Matos; Cees Vermeer; Dina C. Simes; et al. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases. PLOS ONE 2017, 12, e0177829, 10.1371/journal.pone.0177829.
- Carla S.B. Viegas; Lúcia Santos; Anjos Macedo; António Pedro Matos; Ana P. Silva; Pedro L. Neves; An Staes; Kris Gevaert; Rute Morais; C. Vermeer; et al. Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification. Arteriosclerosis, Thrombosis, and Vascular Biology 2018, 38, 575-587, 10.1161/atvbaha.117.310578.
- Alan S. Go; Glenn M. Chertow; Ngjie Fan; Charles E. McCulloch; Chi-Yuan Hsu; Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. New England Journal of Medicine 2004, 351, 1296-1305, 10.1056/nejmoa041031.
- Stein I. Hallan; Brad Astor; S. Romundstad; Knut Aasarød; Kurt Kvenild; Josef Coresh; Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals. Archives of Internal Medicine 2007, 167, 2490, 10.1001/archinte.167.22.2490.
- Brenda Hemmelgarn; Braden Manns; Anita Lloyd; Matthew T. James; Scott Klarenbach; Robert R. Quinn; Natasha Wiebe; Marcello Tonelli; For The Alberta Kidney Disease Network; Relation Between Kidney Function, Proteinuria, and Adverse Outcomes. JAMA 2010, 303, 423, 10.1001/jama.2010.39.
- William G. Goodman; Jonathan Goldin; Beatriz D. Kuizon; Chun Yoon; Barbara Gales; Donna Sider; Yan Wang; Joanie Chung; Aletha Emerick; Lloyd Greaser; et al. Coronary-Artery Calcification in Young Adults with End-Stage Renal Disease Who Are Undergoing Dialysis. New England Journal of Medicine 2000, 342, 1478-1483, 10.1056/NEJM200005183422003.
- Lourdes Craver; M.P. Marco; I. Martinez; Montserrat Rue; M. Borras; M. L. Martin; F. Sarro; José Manuel Valdivielso; E. Fernandez; Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrology Dialysis Transplantation 2007, 22, 1171-1176, 10.1093/ndt/gfl718.
- Mario Cozzolino; Paola Ciceri; Andrea Galassi; Michela Mangano; Stefano Carugo; Irene Capelli; Giuseppe Cianciolo; The Key Role of Phosphate on Vascular Calcification.. Toxins 2019, 11, 213, 10.3390/toxins11040213.
- Xiang Lu; Ming Chang Hu; Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease.. Kidney Diseases 2016, 3, 15-23, 10.1159/000452880.
- Xiang Lu; Ming Chang Hu; Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease.. Kidney Diseases 2016, 3, 15-23, 10.1159/000452880.
- Brecht A. Willems; Malgorzata Furmanik; Marjolein Caron; Martijn L. L. Chatrou; Dennis H. M. Kusters; Tim J. M. Welting; Michael Stock; Marta Rafael; Carla S.B. Viegas; Dina C. Simes; et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling.. Scientific Reports 2018, 8, 4961, 10.1038/s41598-018-23353-y.
- Brecht A. Willems; Malgorzata Furmanik; Marjolein Caron; Martijn L. L. Chatrou; Dennis H. M. Kusters; Tim J. M. Welting; Michael Stock; Marta Rafael; Carla S.B. Viegas; Dina C. Simes; et al. Ucma/GRP inhibits phosphate-induced vascular smooth muscle cell calcification via SMAD-dependent BMP signalling.. Scientific Reports 2018, 8, 4961, 10.1038/s41598-018-23353-y.