The application of porphyrins and their derivatives have been investigated extensively
over the past years for phototherapy cancer treatment. Phototherapeutic Porphyrins have the ability
to generate high levels of reactive oxygen with a low dark toxicity and these properties have made
them robust photosensitizing agents. In recent years, Porphyrins have been combined with various
nanomaterials in order to improve their bio-distribution. These combinations allow for
nanoparticles to enhance photodynamic therapy (PDT) cancer treatment and adding additional
nanotheranostics (photothermal therapy—PTT) as well as enhance photodiagnosis (PDD) to the
reaction. This review examines various porphyrin-based inorganic nanoparticles developed for
phototherapy nanotheranostic cancer treatment over the last three years (2017 to 2020).
Furthermore, current challenges in the development and future perspectives of porphyrin-based
nanomedicines for cancer treatment are also highlighted.
- Porphyrins
- nanotheranostics
- inorganic nanoparticles
- cancer treatment
Cancer Phototherapies with Porphyrin PS-Based Inorganic Nanoparticles
Porphyrin-Based Noble Metallic Nanoparticles
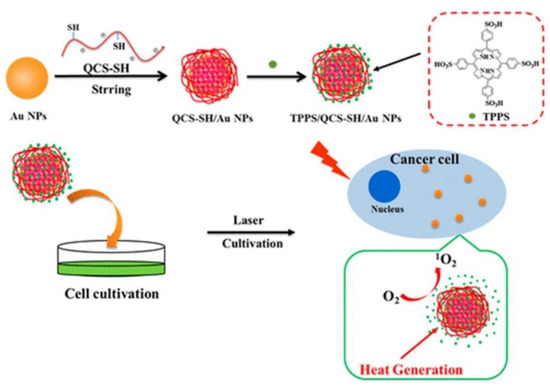
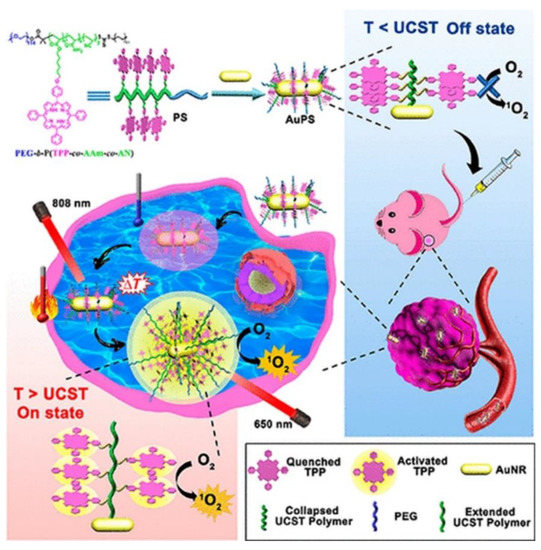
Porphyrin-Based Magnetic Nanoparticles
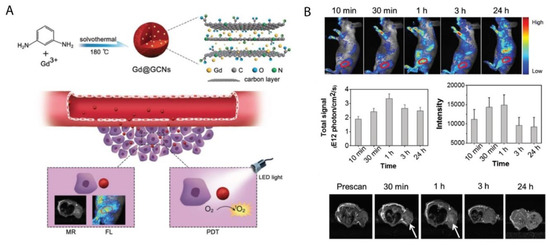
Porphyrin-Based Carbon Nanoparticles
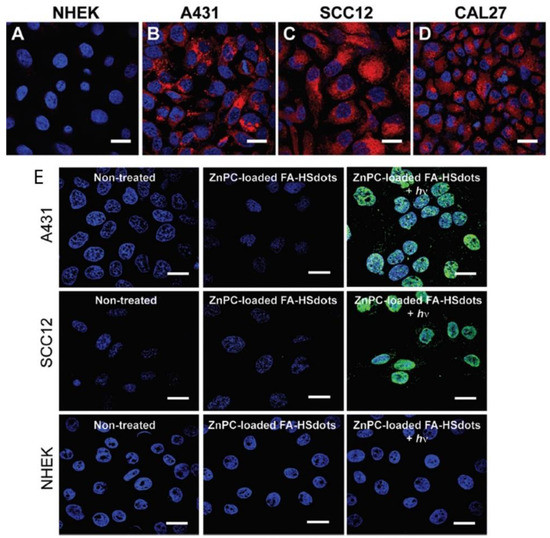
Porphyrin-Based Silica Nanoparticles
Porphyrin-Based Upconversion Nanoparticles
Porphyrin-Based Quantum Dots
Conclusions and Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/ijms21093358
References
- Sasidharan Swarnalatha Lucky; Khee Chee Soo; Yong Zhang; Nanoparticles in photodynamic therapy. Chemical Reviews 2015, 115, 1990-2042, 10.1021/cr5004198.
- Mark E. Davis; Zhuo (Georgia) Chen and Dong M. Shin. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and Technology: A collection of reviews from nature journals; World Scientific: Singapore, Singapore, 2010; pp. 239-250.
- Alyssa Master; Megan Livingston; Anirban Sen Gupta; Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. Journal of Controlled Release 2013, 168, 88-102, 10.1016/j.jconrel.2013.02.020.
- Ron R Allison; Vanderlei S Bagnato; Claudio H Sibata; Future of oncologic photodynamic therapy. Future Oncology 2010, 6, 929-940, 10.2217/fon.10.51.
- Berihun Sisay; Solomon Abrha; Zewdu Yilma; Admassu Assen; Fantahun Molla; Ebisa Tadese; Abrham Wondimu; Naod Gebre-Samuel; Gurudutta Pattnaik; Cancer nanotheranostics: a new paradigm of simultaneous diagnosis and therapy. Journal of Drug Delivery and Therapeutics 2014, 4, 79-86, 10.22270/jddt.v4i5.967.
- H Maeda; J Wu; T Sawa; Y Matsumura; K Hori; Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release 2000, 65, 271-284, 10.1016/s0168-3659(99)00248-5.
- Fenglin Wang; Chengyao Li; Jing Cheng; Zhiqin Yuan; Recent advances on inorganic nanoparticle-based cancer therapeutic agents. International Journal of Environmental Research and Public Health 2016, 13, 1182, 10.3390/ijerph13121182.
- Ncediwe Tsolekile; Simphiwe Nelana; Simphiwe Nelana; Porphyrin as diagnostic and therapeutic agent.. Molecules 2019, 24, 2669, 10.3390/molecules24142669.
- Aisling E. O’Connor; William Gallagher; Annette T. Byrne; Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochemistry and Photobiology 2009, 85, 1053-1074, 10.1111/j.1751-1097.2009.00585.x.
- Nela Malatesti; Ivana Munitic; Igor Jurak; Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophysical Reviews 2017, 9, 149-168, 10.1007/s12551-017-0257-7.
- Shuai Shao; Venugopal Rajendiran; Jonathan F. Lovell; Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coordination Chemistry Reviews 2019, 379, 99-120, 10.1016/j.ccr.2017.09.002.
- Lawrence P. Cook; Greg Brewer; Winnie Wong-Ng; Structural aspects of porphyrins for functional materials applications. Crystals 2017, 7, 223, 10.3390/cryst7070223.
- Quanzheng Zha; Xing Rui; Tiantian Wei; Yongshu Xie; Recent advances in the design strategies for porphyrin-based coordination polymers. CrystEngComm 2014, 16, 7371-7384, 10.1039/C4CE00854E.
- Huijun Phoebe Tham; Hongzhong Chen; Yu Hui Tan; Qiuyu Qu; Sivaramapanicker Sreejith; Lingzhi Zhao; Subbu Venkatraman; Photosensitizer anchored gold nanorods for targeted combinational photothermal and photodynamic therapy. Chemical Communications 2016, 52, 8854-8857, 10.1039/C6CC03076A.
- Yang Hongying; Wang Fuyuan; Zhang Zhiyi; Photobleaching of chlorins in homogeneous and heterogeneous media. Dyes and Pigments 1999, 43, 109-117, 10.1016/s0143-7208(99)00049-2.
- Jinfeng Zeng; Wendi Yang; Dongjian Shi; Xiaojie Li; Hongji Zhang; Mingqing Chen; Porphyrin derivative conjugated with gold nanoparticles for dual-modality photodynamic and photothermal therapies In vitro. ACS Biomaterials Science & Engineering 2018, 4, 963-972, 10.1021/acsbiomaterials.7b00886.
- Gonçalo Doria; Joao Conde; Bruno Veigas; L. Giestas; Carina Almeida; Maria Assunção; João Rosa; Pedro V. Baptista; Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657-1687, 10.3390/s120201657.
- João Conde; Gonçalo Doria; Pedro V. Baptista; Noble metal nanoparticles applications in cancer. Journal of Drug Delivery 2011, 2012, 1-12, 10.1155/2012/751075.
- Ki Young Choi; Gang Liu; Seulki Lee; Xiaoyuan Chen; Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale 2012, 4, 330-342, 10.1039/c1nr11277e.
- Tuan Hiep Tran; Raj Kumar Thapa; Hanh Thuy Nguyen; Tung Thanh Pham; Thiruganesh Ramasamy; Chul Soon Yong; Dong Shik Kim; Jong Oh Kim; Han-Gon Choi; Combined phototherapy in anti-cancer treatment: therapeutics design and perspectives. Journal of Pharmaceutical Investigation 2016, 46, 505-517, 10.1007/s40005-016-0272-x.
- Oriol Penon; María J. Marín; David Russell; Lluisa Perez-Garcia; Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. Journal of Colloid and Interface Science 2017, 496, 100-110, 10.1016/j.jcis.2017.02.006.
- Qiong Yu; Wei-Xia Xu; Ya-Hong Yao; Zeng-Qi Zhang; Shu Sun; Jun Li; Synthesis and photodynamic activities of a new metronidazole-appended porphyrin and its Zn(II) complex. Journal of Porphyrins and Phthalocyanines 2015, 19, 1107-1113, 10.1142/s1088424615500868.
- María E. Alea-Reyes; Jorge Soriano; Inmaculada Mora Espí; Ana Mafalda Nunes Rodrigues; David Russell; Leonardo Barrios; Lluisa Perez-Garcia; Amphiphilic gemini pyridinium-mediated incorporation of Zn(II)meso-tetrakis(4-carboxyphenyl)porphyrin into water-soluble gold nanoparticles for photodynamic therapy. Colloids and Surfaces B: Biointerfaces 2017, 158, 602-609, 10.1016/j.colsurfb.2017.07.033.
- Qingyan Jia; Jiechao Ge; Weimin Liu; Sha Liu; Guangle Niu; Liang Guo; Hongyan Zhang; Pengfei Wang; Gold nanorod@silica-carbon dots as multifunctional phototheranostics for fluorescence and photoacoustic imaging-guided synergistic photodynamic/photothermal therapy. Nanoscale 2016, 8, 13067-13077, 10.1039/c6nr03459d.
- Ying Wang; Feng Zhang; Qian Wang; Piaoping Yang; Huiming Linab; Fengyu Qu; Hierarchical MoSe2 nanoflowers as novel nanocarriers for NIR-light-mediated synergistic photo-thermal/dynamic and chemo-therapy.. Nanoscale 2018, 10, 14534-14545, 10.1039/c8nr04538k.
- Wen‐Xiu Qiu; Li‐Han Liu; Shi‐Ying Li; Qi Lei; Guo-Feng Luo; Xian-Zheng Zhang; ACPI conjugated gold nanorods as nanoplatform for dual image guided activatable photodynamic and photothermal combined therapy In vivo. Small 2017, 13, 1603956, 10.1002/smll.201603956.
- Shen Zhang; Hongying Lv; Jing Zhao; Meng Cheng; Shuqing Sun; Synthesis of porphyrin-conjugated silica-coated Au nanorods for synergistic photothermal therapy and photodynamic therapy of tumor. Nanotechnology 2019, 30, 265102, 10.1088/1361-6528/ab0bd1.
- Gokhan Yilmaz; Bilal Demir; Suna Timur; C. Remzi Becer; Poly(methacrylic acid)-coated Gold nanoparticles: Functional platforms for theranostic applications. Biomacromolecules 2016, 17, 2901-2911, 10.1021/acs.biomac.6b00706.
- Omkara Swami Muddineti; Balaram Ghosh; Swati Biswas; Current trends in using polymer coated gold nanoparticles for cancer therapy. International Journal of Pharmaceutics 2015, 484, 252-267, 10.1016/j.ijpharm.2015.02.038.
- Xuan Wei; Hongzhong Chen; Huijun Phoebe Tham; Nan Zhang; Pengyao Xing; Guangcheng Zhang; Yanli Zhao; Combined photodynamic and photothermal therapy using cross-linked polyphosphazene nanospheres decorated with gold nanoparticles. ACS Applied Nano Materials 2018, 1, 3663-3672, 10.1021/acsanm.8b00776.
- Xuzhe Wang; Jianwei Fu; Minghuan Wang; Yajie Wang; Zhimin Chen; Jianan Zhang; Jiafu Chen; Qun Xu; Facile synthesis of Au nanoparticles supported on polyphosphazene functionalized carbon nanotubes for catalytic reduction of 4-nitrophenol. Journal of Materials Science 2014, 49, 5056-5065, 10.1007/s10853-014-8212-5.
- Minghuan Wang; Jianwei Fu; Zhonghui Chen; Xuzhe Wang; Qun Xu; In situ growth of gold nanoparticles onto polyphosphazene microspheres with amino-groups for alcohol oxidation in aqueous solutions. Materials Letters 2015, 143, 201-204, 10.1016/j.matlet.2014.12.114.
- Ying Hu; Lingjie Meng; Lvye Niu; Qinghua Lu; Facile Synthesis of Superparamagnetic Fe3O4@polyphosphazene@Au Shells for Magnetic Resonance Imaging and Photothermal Therapy. ACS Applied Materials & Interfaces 2013, 5, 4586-4591, 10.1021/am400843d.
- Yang Zhou; Huan Ye; Yongbing Chen; Rongying Zhu; Lichen Yin; Photoresponsive drug/gene delivery systems. Biomacromolecules 2018, 19, 1840-1857, 10.1021/acs.biomac.8b00422.
- Rongying Zhu; Hua He; Yong Liu; Desheng Cao; Jin Yan; Shanzhou Duan; Yongbing Chen; Lichen Yin; Cancer-selective bioreductive chemotherapy mediated by dual hypoxia-responsive nanomedicine upon photodynamic therapy-induced hypoxia aggravation. Biomacromolecules 2019, 20, 2649-2656, 10.1021/acs.biomac.9b00428.
- Kok Chan Chong; Fang Hu; Bin Liu; AIEgen bioconjugates for specific detection of disease-related protein biomarkers. Materials Chemistry Frontiers 2019, 3, 12-24, 10.1039/c8qm00383a.
- Wenpei Fan; Peng Huang; Xiaoyuan Chen; Overcoming the Achilles' heel of photodynamic therapy. Chemical Society Reviews 2016, 45, 6488-6519, 10.1039/C6CS00616G.
- Huanhuan Fan; Guobei Yan; Zilong Zhao; Xiaoxiao Hu; Wenhan Zhang; Hui Liu; Xiaoyi Fu; Ting Fu; Xiao-Bing Zhang; Weihong Tan; et al. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells.. Angewandte Chemie International Edition 2016, 55, 5477-5482, 10.1002/anie.201510748.
- Baoxuan Huang; Jia Tian; Dawei Jiang; Yun Gao; Weian Zhang; NIR-activated “OFF/ON” photodynamic therapy by a hybrid nanoplatform with upper critical solution temperature block copolymers and gold nanorods. Biomacromolecules 2019, 20, 3873-3883, 10.1021/acs.biomac.9b00963.
- Wei Wu; Wouter Driessen; Xiqun Jiang; Oligo(ethylene glycol)-based thermosensitive dendrimers and their tumor accumulation and penetration. Journal of the American Chemical Society 2014, 136, 3145-3155, 10.1021/ja411457r.
- Hans Clevers; The cancer stem cell: premises, promises and challenges. Nature Medicine 2011, 17, 313-319, 10.1038/nm.2304.
- Douglas Hanahan; Robert A Weinberg; Hallmarks of cancer: The next generation. Cell 2011, 144, 646-674, 10.1016/j.cell.2011.02.013.
- Stephanie M. Pyonteck; Leila Akkari; Alberto J. Schuhmacher; Robert L. Bowman; Lisa Sevenich; Daniela F. Quail; Oakley Olson; Marsha L. Quick; Jason T. Huse; Virginia Teijeiro; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine 2013, 19, 1264-1272, 10.1038/nm.3337.
- Yuxun Ding; Jinjian Liu; Yumin Zhang; Xue Li; Hanlin Ou; Tangjian Cheng; Lin Ma; Yingli An; Jianfeng Liu; Fan Huang; et al. A novel strategy based on a ligand-switchable nanoparticle delivery system for deep tumor penetration. Nanoscale Horizons 2019, 4, 658-666, 10.1039/c8nh00415c.
- Stanley B. Brown; Two photons are better than one. Nature Photonics 2008, 2, 394-395, 10.1038/nphoton.2008.112.
- Yizhong Shen; Adam Shuhendler; Deju Ye; Jing-Juan Xu; Huayong Chen; Two-photon excitation nanoparticles for photodynamic therapy. Chemical Society Reviews 2016, 45, 6725-6741, 10.1039/c6cs00442c.
- Shuang Li; Xiaoqin Shen; Qing-Hua Xu; Yong Cao; Gold nanorod enhanced conjugated polymer/photosensitizer composite nanoparticles for simultaneous two-photon excitation fluorescence imaging and photodynamic therapy.. Nanoscale 2019, 11, 19551-19560, 10.1039/c9nr05488j.
- Shuang Liang; Xiaoran Deng; Yun Chang; Chunqiang Sun; Shuai Shao; Zhongxi Xie; Xiao Xiao; Ping'an Ma; Haiyuan Zhang; Ziyong Cheng; et al. Intelligent hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Letters 2019, 19, 4134-4145, 10.1021/acs.nanolett.9b01595.
- Jaber Beik; Ziaeddin Abed; Fatemeh S. Ghoreishi; Samira Hosseini-Nami; Saeed Mehrzadi; Ali Shakeri-Zadeh; Seyed Kamran Kamrava; Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. Journal of Controlled Release 2016, 235, 205-221, 10.1016/j.jconrel.2016.05.062.
- Qian Lv; Huan Min; Dong‐Ban Duan; Wei Fang; Gui‐Ming Pan; Ai-Guo Shen; Qu-Quan Wang; Guangjun Nie; Ji‐Ming Hu; Total aqueous synthesis of Au@Cu2− xS core–shell nanoparticles for in vitro and in vivo SERS/PA imaging‐guided photothermal cancer therapy. Advanced Healthcare Materials 2018, 8, 1801257, 10.1002/adhm.201801257.
- Bo Pang; Yongfeng Zhao; Hannah Luehmann; Xuan Yang; Lisa Detering; Meng You; Chao Zhang; Lei Zhang; Zhi-Yuan Li; Qiushi Ren; et al. 64Cu-doped PdCu@Au tripods: A multifunctional nanomaterial for positron emission tomography and image-guided photothermal cancer treatment. ACS Nano 2016, 10, 3121-3131, 10.1021/acsnano.5b07968.
- Kai Yang; Huan Xu; Liang Cheng; Chunyang Sun; Jun Wang; Zhuang Liu; In vitro and In vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Advanced Materials 2012, 24, 5586-5592, 10.1002/adma.201202625.
- Yijing Liu; Pravin Bhattarai; Zhifei Dai; Xiaoyuan Chen; Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer.. Chemical Society Reviews 2019, 48, 2053-2108, 10.1039/c8cs00618k.
- Xiaoqing Huang; Shaoheng Tang; Xiaoliang Mu; Yan Dai; Guangxu Chen; Zhi-You Zhou; Fangxiong Ruan; Zhilin Yang; Nan-Feng Zheng; Freestanding palladium nanosheets with plasmonic and catalytic properties. Nature Nanotechnology 2010, 6, 28-32, 10.1038/nnano.2010.235.
- Yun Chang; Yan Cheng; Yanlin Feng; Hui Jian; Li Wang; Xiaomin Ma; Xi Li; Haiyuan Zhang; Resonance energy transfer-promoted photothermal and photodynamic performance of gold–copper sulfide yolk–shell nanoparticles for chemophototherapy of cancer. Nano Letters 2018, 18, 886-897, 10.1021/acs.nanolett.7b04162.
- Santana Bala Lakshmanan; Xiaoju Zou; Marius Hossu; Lun Ma; Chang Yang; Wei Chen; Local field enhanced Au/CuS nanocomposites as efficient photothermal transducer agents for cancer treatment.. Journal of Biomedical Nanotechnology 2012, 8, 883-890, 10.1166/jbn.2012.1486.
- Nthabeleng Hlapisi; Tshwafo E Motaung; Linda Z. Linganiso; Oluwatobi S. Oluwafemi; Sandile P. Songca; Encapsulation of gold nanorods with porphyrins for the potential treatment of cancer and bacterial diseases: A critical review.. Bioinorganic Chemistry and Applications 2019, 2019, 27, 10.1155/2019/7147128.
- Xi Wu; Tian Ming; Xin Wang; Peinan Wang; Jianfang Wang; Jiyao Chen; High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2009, 4, 113-120, 10.1021/nn901064m.
- Haifeng Wang; Terry B. Huff; Daniel A. Zweifel; Wei He; Philip S. Low; Alexander Wei; Ji-Xin Cheng; In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proceedings of the National Academy of Sciences 2005, 102, 15752-15756, 10.1073/pnas.0504892102.
- Elodie Boisselier; Didier Astruc; Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews 2009, 38, 1759-1782, 10.1039/b806051g.
- Jelena Levi; Sri Rajasekhar Kothapalli; Te-Jen Ma; Keith Hartman; Butrus T. Khuri-Yakub; Sanjiv Sam Gambhir; Design, synthesis, and imaging of an activatable photoacoustic probe. Journal of the American Chemical Society 2010, 132, 11264-11269, 10.1021/ja104000a.
- Dipanjan Pan; Manojit Pramanik; Angana Senpan; Soumojit Ghosh; Samuel A. Wickline; Lihong V. Wang; Gregory M. Lanza; Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials 2010, 31, 4088-4093, 10.1016/j.biomaterials.2010.01.136.
- Jin Xie; Gang Liu; Henry S. Eden; Hua Ai; Xiaoyuan Chen; Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Accounts of Chemical Research 2011, 44, 883-892, 10.1021/ar200044b.
- Santimukul Santra; Charalambos Kaittanis; Jan Grimm; J. Manuel Perez; Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small 2009, 5, 1862-1868, 10.1002/smll.200900389.
- Xinmeng Liu; Hongli Zhao; Wenxiu Gong; Zhengcheng Zhang; Minbo Lana; Xueling Zhao; Pullulan-functionalized Fe3O4 nanoparticles with mesopore silica-loaded tetraphenylporphyrin tetrasulfonic acid hydrate for targeting photodynamic therapy. Journal of Nanoscience and Nanotechnology 2017, 17, 3880-3887, 10.1166/jnn.2017.13104.
- Ana Margarida Gonçalves Carvalho Dias; A. Hussain; A.S. Marcos; A.C.A. Roque; A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnology Advances 2011, 29, 142-155, 10.1016/j.biotechadv.2010.10.003.
- Jung-Hyun Park; Eun-Wie Cho; Song Yub Shin; Yun- Jung Lee; Kil Lyong Kim; Detection of the asialoglycoprotein receptor on cell lines of extrahepatic origin. Biochemical and Biophysical Research Communications 1998, 244, 304-311, 10.1006/bbrc.1998.8256.
- J C Collins; E Paietta; R Green; A G Morell; R J Stockert; Biotin-dependent expression of the asialoglycoprotein receptor in HepG2.. Journal of Biological Chemistry 1988, 263, 11280–11283, .
- Conroy Sun; Kim Du; Chen Fang; Narayan Bhattarai; Omid Veiseh; Forrest M. Kievit; Zachary Stephen; Nghoon Lee; Richard G. Ellenbogen; Buddy Ratner; et al. PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: Their physicochemical properties and function in vivo. ACS Nano 2010, 4, 2402-2410, 10.1021/nn100190v.
- Taeho Kim; Eric Momin; Jonghoon Choi; Kristy Yuan; Hasan Zaidi; Jaeyun Kim; Mihyun Park; Junyoung Lee; Michael T. McMahon; Alfredo Quiñones-Hinojosa; et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1 contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. Journal of the American Chemical Society 2011, 133, 2955-2961, 10.1021/ja1084095.
- Forrest M. Kievit; Miqin Zhang; Cancer nanotheranostics: Improving imaging and therapy by targeted delivery across biological barriers. Advanced Materials 2011, 23, H217-H247, 10.1002/adma.201102313.
- Inderbir Singh; Ashish K. Rehni; Pradeep Kumar; Manoj Kumar; Hassan Y. Aboul-Enein; Carbon nanotubes: Synthesis, properties and pharmaceutical applications. Fullerenes, Nanotubes and Carbon Nanostructures 2009, 17, 361-377, 10.1080/15363830903008018.
- Nghui Guo; Riku Shibuya; Chisato Akiba; Shunsuke Saji; Takahiro Kondo; Junji Nakamura; Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361-365, 10.1126/science.aad0832.
- Shunhao Wang; Lu Shang; Linlin Li; Yingjie Yu; Chongwei Chi; Kun Wang; Jie Zhang; Run Shi; Heyun Shen; Geoffrey I. N. Waterhouse; et al. Metal-organic-framework-derived mesoporous carbon nanospheres containing porphyrin-like metal centers for conformal phototherapy. Advanced Materials 2016, 28, 8379-8387, 10.1002/adma.201602197.
- Hongmin Chen; Yuwei Qiu; Dandan Ding; Huirong Lin; Wenjing Sun; Geoffrey D. Wang; Weicheng Huang; Weizhong Zhang; Daye Lee; Gang Liu; et al. Gadolinium-encapsulated graphene carbon nanotheranostics for imaging-guided photodynamic therapy. Advanced Materials 2018, 30, 1802748, 10.1002/adma.201802748.
- Enguo Ju; Kai Dong; Zhaowei Chen; Zhen Liu; Chaoqun Liu; Yanyan Huang; Zhenzhen Wang; Fang Pu; Jinsong Ren; Xiaogang Qu; et al. Copper(II)-graphitic carbon nitride triggered synergy: Improved ROS generation and reduced glutathione levels for enhanced photodynamic therapy. Angewandte Chemie 2016, 128, 11639-11643, 10.1002/ange.201605509.
- Yongxia Zhang; Kadir Aslan; Michael J. R. Previte; Chris D. Geddes; Metal-enhanced singlet oxygen generation: A consequence of plasmon enhanced triplet yields. Journal of Fluorescence 2007, 17, 345-349, 10.1007/s10895-007-0196-y.
- Wenting Wu; Liying Zhan; Weiyu Fan; Jizhong Song; XiaoMing Li; Zhongtao Li; Ruiqin Wang; Jinqiang Zhang; Jingtang Zheng; Mingbo Wu; et al. Cu-N dopants boost electron transfer and photooxidation reactions of carbon dots. Angewandte Chemie International Edition 2015, 54, 6540-6544, 10.1002/anie.201501912.
- Dimitrios Tasis; Nikos Tagmatarchis; Alberto Bianco; Maurizio Prato; Chemistry of carbon nanotubes. Chemical Reviews 2006, 106, 1105-1136, 10.1021/cr050569o.
- Andrew Burke; Xuanfeng Ding; Ravi Singh; Robert A. Kraft; Nicole Levi-Polyachenko; Marissa Nichole Rylander; Chris Szot; Cara Buchanan; Jon Whitney; Jessica Fisher; et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proceedings of the National Academy of Sciences 2009, 106, 12897-12902, 10.1073/pnas.0905195106.
- J.-P. Kaiser; M. Roesslein; T. Buerki-Thurnherr; P. Wick; Carbon nanotubes – Curse or blessing. Current Medicinal Chemistry 2011, 18, 2115-2128, 10.2174/092986711795656171.
- Li-Sheng Wang; Min-Chieh Chuang; Ja-an Annie Ho; Nanotheranostics – a review of recent publications. International Journal of Nanomedicine 2012, 7, 4679-4695, 10.2147/IJN.S33065.
- Vahid Shirshahi; Madjid Soltani; Solid silica nanoparticles: applications in molecular imaging. Contrast Media & Molecular Imaging 2015, 10, 1-17, 10.1002/cmmi.1611.
- Dalong Ni; Dawei Jiang; Emily B. Ehlerding; Peng Huang; Weibo Cai; Radiolabeling silica-based nanoparticles via coordination chemistry: Basic principles, strategies, and applications. Accounts of Chemical Research 2018, 51, 778-788, 10.1021/acs.accounts.7b00635.
- Marina Martínez Carmona; R M Lozano; Alejandro Baeza; M. Colilla; María Vallet‐Regí; A novel visible light responsive nanosystem for cancer treatment. Nanoscale 2017, 9, 15967-15973, 10.1039/C7NR05050J.
- Anna M. Sauer; Axel Schlossbauer; Nadia Ruthardt; Valentina Cauda; Thomas Bein; Christoph Bräuchle; Role of endosomal escape for disulfide-based drug delivery from colloidal mesoporous silica evaluated by live-cell imaging. Nano Letters 2010, 10, 3684-3691, 10.1021/nl102180s.
- Dina Aggad; Chiara Mauriello Jimenez; Soraya Dib; Jonas G. Croissant; Laure Lichon; Danielle Laurencin; Sébastien Richeter; Marie Maynadier; Shahad K. Alsaiari; Makhlouf Boufatit; et al. Gemcitabine delivery and photodynamic therapy in cancer cells via porphyrin-ethylene-based periodic mesoporous organosilica nanoparticles. ChemNanoMat 2018, 4, 46-51, 10.1002/cnma.201700264.
- Frank Würthner; Theo E. Kaiser; Chantu R. Saha-Möller; J-aggregates: From serendipitous discovery to supramolecular engineering of functional dye materials. Angewandte Chemie International Edition 2011, 50, 3376-3410, 10.1002/anie.201002307.
- Sanchita Biswas; Hyo-Yang Ahn; Mykhailo V. Bondar; Kevin D. Belfield; Two-photon absorption enhancement of polymer-templated porphyrin-based J-aggregates. Langmuir 2012, 28, 1515-1522, 10.1021/la203883k.
- Duncan Hieu M. Dam; Lingzhi Zhao; Sophia A. Jelsma; Yanli Zhao; Amy S. Paller; Folic acid functionalized hollow nanoparticles for selective photodynamic therapy of cutaneous squamous cell carcinoma. Materials Chemistry Frontiers 2019, 3, 1113-1122, 10.1039/c9qm00144a.
- Dengke Shen; Jianping Yang; Xiaomin Li; Lei Zhou; Renyuan Zhang; Wei Li; Lei Chen; Rui Wang; Fan Zhang; Dongyuan Zhao; et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Letters 2014, 14, 923-932, 10.1021/nl404316v.
- Caroline LeMarchand; Ruxandra Gref; Patrick Couvreur; Couvreur Patrick; Polysaccharide-decorated nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics 2004, 58, 327-341, 10.1016/j.ejpb.2004.02.016.
- D Owensiii; Nicholas A. Peppas; Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics 2006, 307, 93-102, 10.1016/j.ijpharm.2005.10.010.
- Ludovic Bretin; Aline Pinon; Soukaina Bouramtane; Catherine Ouk; Laurence Richard; Marie-Laure Perrin; Alain Chaunavel; Claire Carrion; Frédérique Brégier; Vincent Sol; et al. Photodynamic therapy activity of new porphyrin-xylan-coated silica Nanoparticles in human colorectal cancer.. Cancers 2019, 11, 1474, 10.3390/cancers11101474.
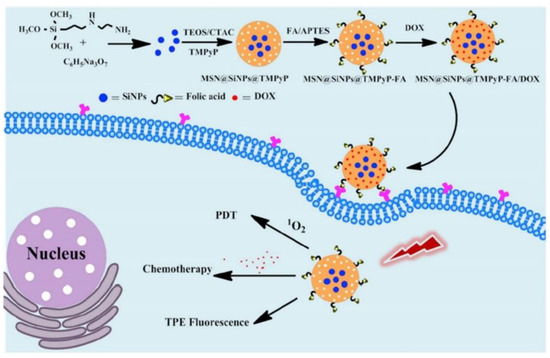
- Si Li; Yue Zhang; Xi- Wen He; Wen-You Li; Yu- Kui Zhang; Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552, 10.1016/j.talanta.2019.120552.
- Shreya Goel; Carolina A. Ferreira; Feng Chen; Paul A. Ellison; Cerise M. Siamof; Todd E Barnhart; Weibo Cai; Todd E. Barnhar; Activatable hybrid nanotheranostics for tetramodal imaging and synergistic photothermal/photodynamic therapy.. Advanced Materials 2018, 30, 1704367, 10.1002/adma.201704367.
- Feng Wang; Debapriya Banerjee; Yongsheng Liu; Zhuo Chen; Xiaogang Liu; Upconversion nanoparticles in biological labeling, imaging, and therapy. The Analyst 2010, 135, 1839-1854, 10.1039/c0an00144a.
- Heike S Mader; Peter Kele; Sayed M Saleh; Otto S. Wolfbeis; Sayed M. Saleh; Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging. Current Opinion in Chemical Biology 2010, 14, 582-596, 10.1016/j.cbpa.2010.08.014.
- Feng Wang; Xiaogang Liu; Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chemical Society Reviews 2009, 38, 976-989, 10.1039/b809132n.
- Ling-Dong Sun; Ye-Fu Wang; Chun-Hua Yan; Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles: Small size and tunable emission/excitation spectra. Accounts of Chemical Research 2014, 47, 1001-1009, 10.1021/ar400218t.
- Annemarie Nadort; Jiangbo Zhao; Ewa M. Goldys; Lanthanide upconversion luminescence at a nanoscale: fundamentals and optical properties. Nanoscale 2016, 8, 13099-13130, 10.1039/c5nr08477f.
- Jun Yao; Cheng Huang; Chaohui Liu; Mei Yang; Upconversion luminescence nanomaterials: A versatile platform for imaging, sensing, and therapy. Talanta 2020, 208, 120157, 10.1016/j.talanta.2019.120157.
- Xiaodan Sun; Peisen Zhang; Yi Hou; Yingying Li; Xiaodan Huang; Zihua Wang; Lihong Jing; Mingyuan Gao; Upconversion luminescence mediated photodynamic therapy through hydrophilically engineered porphyrin. Chemical Engineering and Processing: Process Intensification 2019, 142, 107551, 10.1016/j.cep.2019.107551.
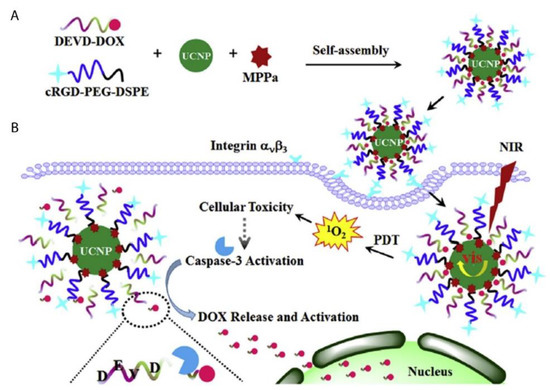
- Na Zhao; Baoyan Wu; Xianglong Hu; Da Xing; NIR-triggered high-efficient photodynamic and chemo-cascade therapy using caspase-3 responsive functionalized upconversion nanoparticles. Biomaterials 2017, 141, 40-49, 10.1016/j.biomaterials.2017.06.031.
- Babak Kateb; Katherine Chiu; Keith L. Black; Vicky Yamamoto; Bhavraj Khalsa; Julia Y. Ljubimova; Hui Ding; Rameshwar Patil; Jose Antonio Portilla-Arias; Michel Modo; et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: What should be the policy?. Neuroimage 2011, 54, S106-S124, 10.1016/j.neuroimage.2010.01.105.
- Yu Cao; Haifeng Dong; Zhou Yang; Xiangmin Zhong; Yi Chen; Wenhao Dai; Xueji Zhang; Aptamer-conjugated graphene quantum dots/porphyrin derivative Theranostic agent for intracellular cancer-related MicroRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Applied Materials & Interfaces 2017, 9, 159-166, 10.1021/acsami.6b13150.
- Ken-Tye Yong; Hong Ding; Indrajit Roy; Wing-Cheung Law; Earl J. Bergey; Anirban Maitra; Paras Prasad; Imaging pancreatic cancer using bioconjugated InP quantum dots. ACS Nano 2009, 3, 502-510, 10.1021/nn8008933.
- Kevin C. Weng; Charles O. Noble; Brigitte Papahadjopoulos-Sternberg; Fanqing F. Chen; Daryl C. Drummond; Dmitri B. Kirpotin; Nghui Wang; Yun K. Hom; Byron Hann; John W. Park; et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Letters 2008, 8, 2851-2857, 10.1021/nl801488u.
- Pavel Zrazhevskiy; Mark Sena; Xiaohu Gao; Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chemical Society Reviews 2010, 39, 4326-4354, 10.1039/b915139g.
- Min-Kyung So; Chenjie Xu; Andreas Loening; Sanjiv Sam Gambhir; Jianghong Rao; Self-illuminating quantum dot conjugates for in vivo imaging. Nature Biotechnology 2006, 24, 339-343, 10.1038/nbt1188.
- Daniel R. Larson; Warren R Zipfel; Rebecca M. Williams; Stephen W. Clark; Marcel Bruchez; Frank W. Wise; Watt W. Webb; Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434-1436, 10.1126/science.1083780.
- Xiaohu Gao; Yuanyuan Cui; Richard M Levenson; Leland W K Chung; Shuming Nie; In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology 2004, 22, 969-976, 10.1038/nbt994.
- Yanyan Fan; Helin Liu; Rongcheng Han; Lu Huang; Hao Shi; Yinlin Sha; Yuqiang Jiang; Extremely high brightness from polymer-encapsulated quantum dots for two-photon cellular and deep-tissue imaging. Scientific Reports 2015, 5, 9908, 10.1038/srep09908.
- Yiming Zhou; Xiaolong Liang; Zhifei Dai; Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 2016, 8, 12394-12405, 10.1039/c5nr07849k.
- Xiangdong Xue; Aaron Lindstrom; Yuanpei Li; Porphyrin-based nanomedicines for cancer treatment. Bioconjugate Chemistry 2019, 30, 1585-1603, 10.1021/acs.bioconjchem.9b00231.
- Charles J. Gomer; Nicholas J. Razum; Acute skin response in albino mice following porphyrin photosensitization under oxic and anoxic conditions. Photochemistry and Photobiology 1984, 40, 435-439, 10.1111/j.1751-1097.1984.tb04614.x.
- Xiaoju Zou; Mingzhen Yao; Lun Ma; Marius Hossu; Xiumei Han; Petras Juzenas; Wei Chen; X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339-2351, 10.2217/nnm.13.198.
- Samana Shrestha; Jing Wu; Bindeshwar Sah; Adam Vanasse; Leon N Cooper; Lun Ma; Gen Li; Huibin Zheng; Wei Chen; Michael P. Antosh; et al. X-ray induced photodynamic therapy with copper-cysteamine nanoparticles in mice tumors.. Proceedings of the National Academy of Sciences 2019, 116, 16823-16828, 10.1073/pnas.1900502116.
- Benjamin Cline; Ian Delahunty; Jin Xie; Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2018, 11, e1541, 10.1002/wnan.1541.
- Wen Song; Jing Kuang; Chu-Xin Li; Mingkang Zhang; Diwei Zheng; Xuan Zeng; Chuanjun Liu; Xian-Zheng Zhang; Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano 2018, 12, 1978-1989, 10.1021/acsnano.7b09112.
- Guangxu Lan; KaiYuan Ni; ZiWan Xu; Samuel S. Veroneau; Yang Song; Wenbin Lin; Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. Journal of the American Chemical Society 2018, 140, 5670-5673, 10.1021/jacs.8b01072.
- Chunbai He; Xiaopin Duan; Nining Guo; Christina Chan; Christopher Poon; Ralph R. Weichselbaum; Wenbin Lin; Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications 2016, 7, 1-12, 10.1038/ncomms12499.
