Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer is the most common breast cancer subtype, and endocrine therapy (ET) remains its therapeutic backbone. Although anti-estrogen therapies are usually effective initially, approximately 50% of HR+ patients develop resistance to ET within their lifetime, ultimately leading to disease recurrence and limited clinical benefit. The recent addition of cyclin-dependent kinase 4 (CDK4) and CDK6 inhibitors (palbociclib, ribociclib, abemaciclib) to ET have remarkably improved the outcome of patients with HR+ advanced breast cancer (ABC) compared with anti-estrogens alone, by targeting the cell-cycle machinery and overcoming some aspects of endocrine resistance.
- CDK4/6 inhibitors
- breast cancer
- endocrine therapy (ET)
- advanced breast cancer (ABC)
- endocrine resistance
1. Introduction
Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer is the most common breast cancer subtype, and endocrine therapy (ET) remains its therapeutic backbone. Although anti-estrogen therapies are usually effective initially, approximately 50% of HR+ patients develop resistance to ET within their lifetime, ultimately leading to disease recurrence and limited clinical benefit [1]. The recent addition of cyclin-dependent kinase 4 (CDK4) and CDK6 inhibitors (palbociclib, ribociclib, abemaciclib) to ET have remarkably improved the outcome of patients with HR+ advanced breast cancer (ABC) compared with anti-estrogens alone, by targeting the cell-cycle machinery and overcoming some aspects of endocrine resistance.
2. Mechanism of Endocrine Therapy Resistance
Until now, three distinct pathways of regulation of estrogen receptor (ER) gene (ESR1) expression were thought to be mainly involved in breast cancer resistance to ET [2]: (i) Classic signaling: ligand-binding domain mutations in the ER that activates ESR1 expression (approximately 18% of endocrine-resistant HR+ breast cancers); (ii) Ligand independent signaling: ER can also be activated as a consequence of signaling events downstream of receptor tyrosine kinases (RTKs); (iii) Non-genomic mechanisms: signaling can be mediated by ER that is localized at the cell membrane or in the cytoplasm of breast cancer cells. A figure that summarizes all the described endocrine-resistance mechanisms was reported. (Figure 1)
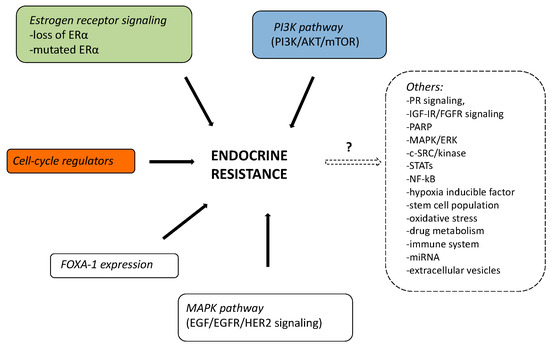
Figure 1. Possible mechanisms of endocrine resistance in summary.
2.1. Mutations of ER-α
ER mutations are rare in primary tumors but appear to be reasonably frequent in the progression to endocrine resistance [3]. The spot mutations drive estrogen-independent transcriptional activity and cancer cell proliferation, leading to endocrine resistance [4].
2.2. Loss of ER-α
Lack of ER is one of the principal causes of de novo resistance to ET. The loss of ER-α expression can be achieved by epigenetic mechanisms such as methylation of CpG islands or histone deacetylase activity in the ESR1 promoter; DNA methyltransferase (DMNT) and histone deacetylase (HDAC) influence chromatin condensation regulating the ER gene expression [5].
In vitro experiments showed that DNMT1 inhibitors (Aza) and HDAC inhibitors (TSA) reduce chromatin condensation leading to ER expression in ER-negative breast cancer cells [6]. Moreover, AZA + TSA treatment inhibits tumor growth in mice inoculated with ER-negative breast cancer cells after ovarian ablation and restores sensitivity to tamoxifen [7].
2.3. MAPK Pathway (EGF/EGFR/HER2 Signaling)
Mutation in the MAPK pathway has been reported in approximately 13% of breast cancers [1]. In addition to the expected ESR1 hotspot mutations, ERBB2 and NF1 were the genes, mostly mutually exclusive, with the greatest difference in mutational frequency between pre- and post-hormonal therapy for HR + HER2− breast cancers [1]. Tamoxifen-resistant breast cancer cells (LTam) showed an hyperactivation of the HER/EGFR/Akt/ERK pathway. An in vitro study demonstrated that, by using lapatinib, a dual inhibitor of EGFR and HER2, tamoxifen sensitivity of LTam cells was restored [8].
2.4. PI3K Pathway (PI3K/AKT/mTOR)
Resistance to letrozole in breast cancer cells is associated with hyperactivation of p70S6K and AKT, which are involved in the PI3K pathway. PI3K inhibitor (BEZ235, AEW541), mTOR inhibitor (RAD001), and EGFR/HER2 inhibitor (lapatinib) suppress proliferation of letrozole-resistant breast cancer cells [9]. PI3K inhibition enhances ER function and the response to endocrine therapies. Indeed, the PI3K inhibitor alpelisib (BYL719) in combination with the ER inhibitor fulvestrant has profound antitumor activity both in vivo and in vitro [10].
2.5. FOXA-1 Expression
Foxa-1 is an essential protein for the transcriptional activity of both ER and androgen receptor (AR). The induction of FOXA-1 expression with doxycycline in breast cancer cells was directly related to a high level of expression of proliferation genes and inversely to estrogen sensitivity genes. Moreover, increased expression of FOXA1 contributes to tumor aggressiveness and endocrine resistance [11].
Other genomic and nongenomic mechanisms of resistance to ET are under investigation (eg, progesterone receptor signaling, IGF-IR, FGFR signaling, PARP, MAPK/ERK, c-SRC/KINASE, STATs, NF-kB, hypoxia inducible factor, stem cell population, oxidative stress, drug metabolism, immune system, miRNA, and extracellular vesicles) although the precise mechanisms remain largely unexplained. Complicating matters, some patients with ABC have distinct and coexisting mechanisms of resistance to ET in distinct tumor subclones that cannot be captured by a single biopsy of a metastatic site. Rizavi et al. [1] suggest that there was an emerging taxonomy of endocrine-resistant breast cancer, but some of these alterations were a consequence of selective therapeutic pressure and mechanisms of systemic therapy resistance. Therefore, to better define the complexity of endocrine resistance in HR+, HER- ABC, further genomic study of a large cohort of clinically phenotyped patients is needed.
2.6. Cell-Cycle Regulators and Endocrine Resistance
Activation by D-type cyclin proteins leading to phosphorylation of retinoblastoma-associated protein and E2F protein-mediated transcription of cell-cycle genes, such as cyclins A and E, are critical for cell-cycle progression. Therefore, the action of cyclin-dependent kinases 4/6 (CDK4/6) by regulating the transition from G1-to-S cell-cycle phase is crucial for normal and cancer cell proliferation [12].
Indeed, CDK4/6 inhibitors have shown significant preclinical activity in ER-positive breast cancer, especially when combined with anti-estrogen therapy. In an in vitro experiment, CDK4 inhibitor (PD-0332991) reduced cell tumor growth of fulvestrant-insensitive ER+ cell lines and tumor growth of mice bearing an ER+ breast cancer cell line [13].
On transcriptome analysis, 58 tumor samples from letrozole-resistant patients were enriched for cell-cycle related genes. Treatment with palbociclib compared with fulvestrant significantly downregulated the expression of cell-cycle genes associated with letrozole resistance [14].
Abemaciclib is also a highly selective, reversible CDK 4/6 inhibitor with the highest half maximal inhibitory concentrations of 2 nM and 10 nM for CDK4 and CDK6, respectively [15].
In addition, ribociclib showed remarkable preclinical efficacy in ER+ BC mouse models by reducing tumor growth both as a single agent and in combination with letrozole or fulvestrant and with a PI3K inhibitor.
Although the three approved CDK4/6 inhibitors—palbociclib, ribociclib, and abemaciclib—seem to have essentially overlapping patterns of activity, as multikinase inhibitors, they could have many other mechanisms of action on several cellular populations other than tumor cells, particularly in the bone microenvironment. The extent to which these off-target events occur may also explain the difference in survival reported with the three different CDK4/6 inhibitors, but their significance in the overall treatment of disease is still not clear [16].
3. Clinical Implications
At the 4th ESO-ESMO international consensus, primary endocrine resistance was defined as “relapse while on the first 2 years of adjuvant ET, or PD within first 6 months of first-line ET for ABC, while on ET” and secondary endocrine resistance as “relapse while on adjuvant ET but after the first 2 years, or relapse within 12 months of completing adjuvant ET, or PD ≥ 6 months after initiating ET for ABC, while on ET”. However, these definitions are subsequent to CDK4/6 inhibitors trials and limited to 67% consensus.
According to endocrine sensitivity/resistance, four main scenarios are represented among the phase 3 CDK4/6-based trials: (1) de novo metastatic disease; (2) late relapse; (3) early relapse; and (4) second line (Table 1).
Table 1. CDK4/6 inhibitors phase 3 trials according to endocrine sensitivity/resistance patients representation and outcome results.
| Drug | Trial | Setting | Endocrine Sensitivity/Resistance (%) | Efficacy | Adverse Events of Interest | ||||
|---|---|---|---|---|---|---|---|---|---|
| De Novo | Late Relapse | Early Relapse | Second Line | PFS (Months) | OS (Months) | ||||
|
Ribociclib |
MONALEESA-2 [17] |
First line |
34 |
64.7 |
— |
— |
RIBO + LET: 25.3 PBO + LET: 16.0 (HR, 0.56; 95% CI, 0.43–0.72; p < 0.001) |
Immature |
|
|
MONALEESA-7 [18] |
First and second line |
40 |
52.5 |
— |
14 (after CT) |
RIBO + TAM/NSAI: 23.8 months PBO + TAM/NSAI: 13.0 months (HR, 0.55; 95% CI, 0.44–0.69; p < 0.0001) |
HR, 0.712; 95% CI, 0.535–0.948; p = 0.00973 |
|
|
|
MONALEESA-3 [19] |
First and second line |
20 |
29 |
28 |
20 |
RIB + FUL: 20.5 (33.6 in first line) PBO + FUL: 12.8 (19.2 in first line) (HR, 0.593; 95% CI, 0.480–0.732; p < 0.001) |
HR, 0.724; 95% CI, 0.568– 0.924; p = 0.00455 |
|
|
|
Abemaciclib |
MONARCH-3 [20] |
First line |
41.2 |
58.8 |
— |
— |
ABE + NSAI: 28.18 months PBO + NSAI: 14.76 months (HR, 0.540; 95% CI, 0.418–0.698; p = 0.000002) |
Immature |
|
|
MONARCH-2 [21] |
Second line |
— |
— |
60 |
38 |
ABE + FUL: 16.4 PBO + FUL: 9.3 (HR, 0.553; 95% CI, 0.449–0.681; p < 0.001) |
HR, 0.757; 95% CI, 0.606–0.945; p = 0.0137 |
|
|
|
Palbociclib |
PALOMA-2 [22] |
First line |
37.6 |
40.01 |
— |
— |
PAL + LET: 24.8 PBO + LET: 14.5 (HR, 0.58; 95% CI, 0.46–0.72; p <0.001) |
Immature |
|
|
Second line |
— |
— |
21 |
79 |
PAL + FUL: 9.5 PBO + FUL: 4.6 (HR, 0.46; 95% CI, 0.36–0.59; p < 0.0001) |
HR, 0.81; 95% CI, 0.64–1.03; p = 0.09 |
|
||
Abbreviations: AE, adverse event; PFS, progression-free survival; HR, hazard ratio; RIBO, ribociclib; LET, letrozole; PBO, placebo; FUL, fulvestrant; PAL, palbociclib; TAM, tamoxifen; NSAI, nonsteroidal aromatase inhibitor; TE, thromboembolic event.
3.1. First Line
According to the literature data, international clinical guidelines recommended ET as the preferred option for HR+, HER2− ABC in as first-line therapy, even in the presence of visceral disease.
Chemotherapy is reserved for visceral crisis, defined as severe organ dysfunction and rapid progression of disease, or progression on multiple lines of ET. A recent metanalysis showed that no chemotherapy regimen with or without targeted therapy is significantly better than CDK4/6 inhibitors plus hormone therapies in terms of progression-free survival [26]. Thus, considering the significant improvement in the outcome of patients with HR+, HER2− ABC with adjunct of CDK4/6 inhibitor to standard hormonal therapies compared with ET alone, the combinatorial strategy of CDK4/6 inhibitors and ET should be considered as the new standard of care in first- or second-line therapy (Table 1). A network meta-analysis, including patients treated with CDK4/6 inhibitors combined with aromatase inhibitors (AIs) or fulvestrant in comparison with AI or fulvestrant monotherapy, confirmed CDK4/6 inhibitors had similar efficacy when associated with an AI in the first-line treatment of HR+ ABC, and were superior to either fulvestrant or AI monotherapy, regardless of any other patient or tumor characteristics [27].
In de novo patients, all the CDK4/6 inhibitors performed better than ET alone in terms of progression-free survival (PFS) (MONALEESA-2, hazard ratio, 0.45; MONALEESA-3, hazard ratio, 0.57; MONALEESA-7, hazard ratio, 0.43; MONARCH-3, hazard ratio, 0.54; PALOMA-2, hazard ratio, 0.67) [17][18][19][20][21][22]. The MONALEESA-7 trial was the only phase 3 trial to study CDK4/6 inhibitors as first-line therapy in a premenopausal population; the percentages of premenopausal patients studied with palbociclib and abemaciclib derived from the second-line trials, PALOMA-3 and MONARCH-2, were 20.7% and 16.1%, respectively, where CDK4/6 inhibitors were combined with fulvestrant. The updated analysis of MONALEESA-7 showed that the addition of ribociclib to ET significantly prolonged overall survival (OS) compared with ET alone with an estimated OS at 42 months of 70.2% in the ribociclib group and 46.0% in the placebo group (hazard ratio, 0.71; p = 0.00973) [28]. On the basis of its innovative results, ribociclib plus ET (AI/TAM) with ovarian function suppression (OFS) was recently approved by the Italian Medicines Agency (AIFA), and thus it could be considered as the preferred first-line treatment option in premenopausal patients with HR+, HER2− ABC. The overall response rate (ORR) was similar in MONALEESA-2 (ORR = 52.7%), MONARCH-3 (ORR= 59.2%), and PALOMA 2 (ORR = 55.3%). However, with a median PFS of 33.6 months rather than 22–28 months with palbociclib or with abemaciclib plus AI, and a relative risk reduction in death of 28% in the first-line setting of MONALEESA-3, ribociclib plus fulvestrant seems to be the preferred first-line treatment option in postmenopausal patients [19]. Moreover, whereas the data on OS for palbociclib and abemaciclib are still immature or are from real-world data of retrospective studies [29][30], the data on OS with ribociclib come directly from phase 3 trials [18][19].
The three CDK4/6 inhibitors reported a good toxicity profile; there was higher incidence of grade 3–4 neutropenia with both ribociclib and palbociclib, and diarrhea and abdominal pain with abemaciclib (Table 1). QTcF prolongation with ribociclib occurred in no more than 23 patients (7% of cases) in the MONALEESA-7 trial. However, the percentage of cases of QT prolongation was even lower in clinical practice. Dose reductions due to adverse events was reported in 54.5%, 33.1%, and 31% of cases in MONALEESA-2, MONALEESA-3, and MONALEESA-7, without any significant impact on PFS [17][18][19]. Thus, if toxicity occurs, the dosage of ribociclib can be reduces without affecting its efficacy. Both MONARCH [20][21] and PALOMA-3 [24] studies showed no difference in PFS for patients who had the dose reduced due to any adverse events compared with those who did not.
However, the clinical scenario could be much more complex according to potential drug-drug interactions (DDIs) in patients with breast cancer treated with CDK4/6 inhibitors; DDIs may occur in patients who take polypharmacy [31]. Therefore, better knowledge of how patient metabolism and DDIs could affect both the efficacy and safety of CDK4/6 inhibitors should always be considered to maximize the personalization of cancer care in patients with ABC (Figure 2). Indeed, any physician should know that concomitant medications (e.g., proton pump inhibitors and corticosteroids), pharmacogenetic profile, and pathophysiological conditions could influence absorption, distribution, metabolism, and elimination pharmacokinetics. A personalized therapeutic approach taking into consideration all these factors potentially contributing to an altered pharmacokinetic/pharmacodynamic profile could better drive safe and effective clinical use of third generation CDK4/6 inhibitors [32]. According to the application of precision medicine in the management of cancer treatment, new software, Drug-PIN, which combines data regarding DDIs and the pharmacogenomic profile of cancer patients, is under investigation at our institution [33].
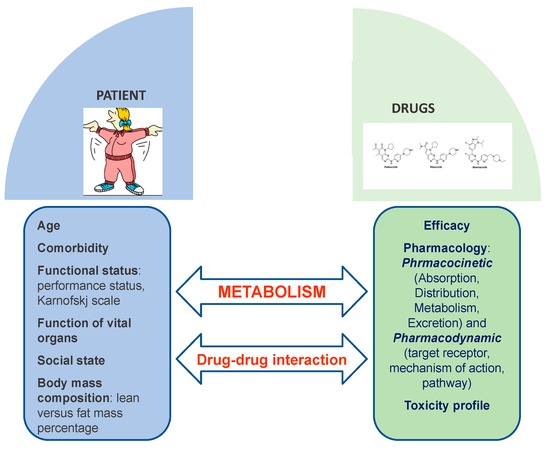
Figure 2. The complexity of patient metabolism and potential drug-drug-interactions.
3.2. Second Line and Early Relapse
According to the Associazione Italiana di Oncologia Medica (AIOM) guidelines, “early relapse” is defined as aggressive disease that presents itself with a short disease-free interval from the adjuvant therapy (progression during or within 12 months from the end of adjuvant ET); this it is slightly different from the European Society for Medical Oncology definitions of primary and secondary resistance mentioned earlier, but it is the same definition in the three different trials:
MONALEESA-3: Patients who had a relapse during or within 12 months after completion of adjuvant or neoadjuvant ET.
MONARCH-2: Patients were required to have progressive disease while receiving neoadjuvant or adjuvant ET, within 12 months from the end of adjuvant ET.
PALOMA-3: Disease relapse or progression had to occur while on or within 12 months of completion of adjuvant therapy irrespective of menopausal status.
All these trials enrolled patients who experienced progression after mono-ET (with tamoxifen or aromatase inhibitor) and their primary endpoint was the difference in survival between CDK4/6 inhibitor plus fulvestrant versus fulvestrant plus placebo.
With the except of MONALEESA-3, both MONARCH-2, and PALOMA-3 included pre- and postmenopausal women [19][21][23][24]. According to 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) [34], young women with ER+ ABC should have adequate ovarian suppression or ablation (OFS/OFA) and then be treated in the same way as postmenopausal women with endocrine agents with or without targeted therapies. Since the first step is to render the patient postmenopausal, all treatment recommendations should be common to both post and premenopausal patients. Therefore, patients should be informed on the options of ovarian ablation by laparoscopic bilateral oophorectomy so that it provides definitive estrogen suppression and contraception, avoids potential initial tumor flare with a luteinizing hormone-releasing hormone agonist, and may increase eligibility for clinical trials. However, no significant difference in median OS was found between premenopausal or perimenopausal patients included in PALOMA-3 (108 patients [21%], hazard ratio, 1.07; 95% confidence interval [CI], 0.61–1.86) [35] and MONARCH-2 (114 patients [17%], hazard ratio, 0.68; 95% CI, 0.37–1.25) [21] Otherwise, median PFS and ORR were significantly higher with ribociclib than with placebo in the subgroup analysis of premenopausal patients treated previously with chemotherapy for ABC included in the MONALEESA-7 trial [36].
In the indirect comparison of the populations enrolled in the three phase 3 trials with CDK4/6 inhibitors and fulvestrant (Table 2), the PFS data were similar but the results on OS were slightly different, probably not only due to the intrinsic pharmacokinetic differences between ribociclib, palbociclib, and abemaciclib [31] but also due to the different characteristics of the patient populations (Table 2).
Table 2. Phase 3 trials of CDK4/6 inhibitors that included patients(n) in second-line and early relapse settings, in respect to the total populations enrolled (N).
| Trial | Population | n/N | Treatment | PFS (Months) | OS (Months) | ORR (%) |
|---|---|---|---|---|---|---|
|
MONALEESA-3 |
|
346/726 |
Ribociclib plus fulvestrant versus placebo + fulvestrant |
14.6 (HR, 0.57) |
40.2 (HR, 0.73) |
40.9 |
|
MONALEESA-7 |
|
94/672 |
Ribociclib plus ET plus goserelin versus placebo + ET and goserelin |
16.6 (HR 0.54) |
NR (HR 0.67) |
26 |
|
MONARCH-2 |
|
669 |
Abemaciclib plus fulvestrant versus placebo + fulvestrant |
16.4 (HR, 0.55) |
46.7 (HR 0.75) |
48.1 |
|
PALOMA-3 |
|
521 |
Palbociclib plus fulvestrant versus placebo + fulvestrant |
11.2 (HR, 0.50) |
34.9 (HR, 0.81; NS, p = 0.09) |
25 |
Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, overall response rate; HR, hazard ratio; ET, endocrine therapy; NS, not significant.
For example, PALOMA-3 included patients pretreated with more than one line of therapy compared with patients included in MONALEESA-3 and MONARCH-2, where only one previous line of ET was allowed [19][21][23].
In patients with previous ET, the median PFS was significantly better in the CDK4/6 arm in MONALEESA-3, MONARCH-2, and PALOMA-3. However, in postmenopausal women pretreated with ET and postmenopausal women with early relapse, the median OS reached 40.2 months in the ribociclib group compared with 32.5 months in the placebo group (hazard ratio, 0.73; 95% CI, 0.53–1.00) [19].
In the MONARCH-2 early relapse group, median OS was improved by 9.4 months, with a median OS of 46.7 months in the abemaciclib arm and 37.3 months in the placebo arm (hazard ratio, 0.757; 95% CI, 0.606–0.945; p = 0.01) [21]. However, earlier separation of the curves and a numerically larger effect were observed in patients with primary ET resistance (hazard ratio, 0.686; 95% CI, 0.451–1.043) compared with patients with secondary ET resistance (hazard ratio, 0.787; 95% CI, 0.606–1.021) but no statistically significant interaction was observed.
In the PALOMA-3 trial, the median OS was not statistically significant (34.9 months in the palbociclib-fulvestrant group and 28.0 months in the placebo-fulvestrant group; HR, 0.81; 95% CI, 0.64–1.03; p = 0.09) [24]. However, women were enrolled regardless of menopausal status and including patients treated with more than one line of previous ET. The difference in median OS was statistically significant (39.7 months in the palbociclib group and 29.7 months in the placebo group; hazard ratio, 0.72) only among those patients with documented sensitivity (secondary resistance) to previous ET.
These results highlight the following considerations: (1) ribociclib seems to perform better in an acquired resistance setting; (2) abemaciclib seems to perform better in the primary endocrine resistance setting; (3) despite the promising results with palbociclib in pretreated patients, the data on OS are still inconclusive. Overall, the adjunct of CDK4/6 inhibitors in patients with visceral disease versus patients who did not have visceral metastasis was beneficial in the three trials.
Finally, the retrospective analysis of second-line treatment in patients who progressed on CDK4/6 inhibitors as first-line therapy also deserves mention. In a recent study among patients who progressed on palbociclib (n = 104), the most frequent next-line treatment was capecitabine (n = 21), followed by eribulin (n = 16), nab-paclitaxel (n = 15), and exemestane plus everolimus (n = 12). The median PFS with hormonal therapy or combinations (n = 32) after first-, second-, and subsequent-line palbociclib was 17.0, 9.3, and 4.2 months, respectively (p = 0.04); whereas the median PFS with chemotherapy (n = 70) was not reached at 4.7 and 4.1 months in patients after first-, second-, or subsequent-line therapy with palbociclib (p = 0.56). The authors concluded that in real-world practice, hormone therapy alone or in combination with targeted agents remains an effective option after palbociclib progression [37].
In a retrospective analysis of patients treated according to the BOLERO-2 trial with everolimus plus exemestane, 17 patients had undergone previous CDK4/6 inhibitor therapy and 16 had not. In this study, there was no significant difference in PFS (median, 5.7 months versus 4.7 months, p = 0.890) or OS (median, 17.8 months versus 11.4 months, p = 0.177) between patients who received previous therapy with CDK4/6 inhibitors and those who did not, respectively. Therefore, the combination of everolimus plus exemestane remains a good option in advanced lines of treatment [38].
Moreover, a retrospective study of 58 patients with HR+/HER2− ABC who received abemaciclib after disease progression on palbociclib was conducted. In this study, 20 patients (34%) received sequential courses of therapy, and 38 patients (66%) had at least one intervening non-CDK4/6 inhibitor regimen. Fourteen patients (24%) received abemaciclib monotherapy and 44 patients (76%) received abemaciclib in combination with an anti-estrogen, including fulvestrant (52%), an aromatase inhibitor (22%), and tamoxifen (2%) [39]. In this analysis, 20 patients (34%) had early disease progression (duration < 90 days), whereas 21 patients (36%) had treatment duration exceeding 6 months, including 10 who remained on treatment at the interim analysis (range, 181–413 days). The median PFS was 5.8 months (95% CI, 3.4–8.0). Although the results are not conclusive, this is the first multicenter experience to demonstrate that a substantial proportion of patients continue to maintain clinical benefit with another CDK4/6 inhibitor after previous CDK4/6 inhibitor, highlighting the potential for their use after CDK4/6 blockade.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13020332
References
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643.
- Murphy, C.G.; Dickler, M.N. Endocrine resistance in hormone-responsive breast cancer: Mechanisms and therapeutic strategies. Endocr. Relat. Cancer 2016, 23, 337–352.
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445.
- Hayes, E.L.; Lewis-Wambi, J.S. Mechanisms of endocrine resistance in breast cancer: An overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015, 17, 40.
- Giacinti, L.; Claudio, P.P.; Lopez, M.; Giordano, A. Epigenetic Information and Estrogen Receptor Alpha Expression in Breast Cancer. Oncologist 2006, 11, 1–8.
- Fan, A.X.C.; Radpour, R.; Haghighi, M.M.; Kohler, C.; Xia, P.; Hahn, S.; Holzgreve, W.; Zhong, X.Y. Mitochondrial DNA content in paired normal and cancerous breast tissue samples from patients with breast cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 983–989.
- Leary, A.F.; Drury, S.; Detre, S.; Pancholi, S.; Lykkesfeldt, A.E.; Martin, L.A.; Dowsett, M.; Johnston, S.R.D. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin. Cancer Res. 2010, 16, 1486–1497.
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413.
- Bosch, A.; Li, Z.; Bergamaschi, A.; Ellis, H.; Toska, E.; Prat, A.; Tao, J.J.; Spratt, D.E.; Viola-Villegas, N.T.; Castel, P.; et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med. 2015, 7, 283ra51.
- Fu, X.; Jeselsohn, R.; Pereira, R.; Hollingsworth, E.F.; Creighton, C.J.; Li, F.; Shea, M.; Nardone, A.; De Angelis, C.; Heiser, L.M.; et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6600–E6609.
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430.
- Miller, T.W.; Balko, J.M.; Fox, E.M.; Ghazoui, Z.; Dunbier, A.; Anderson, H.; Dowsett, M.; Jiang, A.; Adam Smith, R.; Maira, S.M.; et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011, 1, 338–351.
- Guerrero-Zotano, A.L.; Stricker, T.P.; Formisano, L.; Hutchinson, K.E.; Stover, D.G.; Lee, K.M.; Schwarz, L.J.; Giltnane, J.M.; Estrada, M.V.; Jansen, V.M.; et al. ERþ Breast cancers resistant to prolonged neoadjuvant letrozole exhibit an e2f4 transcriptional program sensitive to cdk4/6 inhibitors. Clin. Cancer Res. 2018, 24, 2517–2529.
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837.
- Chen, P.; Lee, N.V.; Hu, W.; Xu, M.; Ferre, R.A.; Lam, H.; Bergqvist, S.; Solowiej, J.; Diehl, W.; He, Y.A.; et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 2016, 15, 2273–2281.
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547.
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915.
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Val Bianchi, G.; Esteva, F.J.; Martín, M.; et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472.
- Goetz, M.P.; Toi, M.; Campone, M.; Trédan, O.; Bourayou, N.; Sohn, J.; Park, I.H.; Paluch-Shimon, S.; Huober, J.; Chen, S.C.; et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646.
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884.
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35.
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Bartlett, C.H.; Zhang, K.; et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2015, 373, 209–219.
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018, 379, 1926–1936.
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phas. Lancet Oncol. 2016, 17, 425–439.
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369.
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nisticò, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661.
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316.
- DeMichele, A.; Cristofanilli, M.; Brufsky, A.; Liu, X.; Mardekian, J.; McRoy, L.; Layman, R.M.; Rugo, H.S.; Finn, R.S. Overall Survival for First-Line Palbociclib Plus Letrozole vs Letrozole Alone for HR+/HER2–Metastatic Breast Cancer Patients in US Real-World Clinical Practice. Available online: Sabcs.posterview.com (accessed on 20 December 2020).
- Carter, G.C.; Sheffield, K.M.; Gossai, A.; Huang, Y.-J.; Zhu, Y.E.; Bowman, L.; Smith, E.N.; Mathur, R.; Cohen, A.B.; Baxi, S.; et al. Initial real world treatment patterns and outcomes of Abemaciclib for the treatment of HR+, HER2-metastatic breast cancer. Cancer Res. 2020, 80.
- Fogli, S.; Del Re, M.; Curigliano, G.; Van Schaik, R.H.; Lancellotti, P.; Danesi, R. Complications of Treatment Drug-drug interactions in breast cancer patients treated with CDK4/6 inhibitors. Cancer Treat. Rev. 2019, 74, 21–28.
- Roncato, R.; Angelini, J.; Pani, A.; Cecchin, E.; Sartore-Bianchi, A.; Siena, S.; De Mattia, E.; Scaglione, F.; Toffoli, G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int. J. Mol. Sci. 2020, 21, 6350.
- Roberto, M.; Rossi, A.; Panebianco, M.; Pomes, L.M.; Arrivi, G.; Ierinò, D.; Simmaco, M.; Marchetti, P.; Mazzuca, F. Drug–Drug Interactions and Pharmacogenomic Evaluation in Colorectal Cancer Patients: The New Drug-PIN® System Comprehensive Approach. Pharmaceuticals 2021, 14, 67.
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657.
- Turner, N.C.; Liu, Y.; Zhu, Z.; Loi, S.; Colleoni, M.; Loibl, S.; DeMichele, A.; Harbeck, N.; André, F.; Bayar, M.A.M.; et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2019, 37, 1169–1178.
- Hurvitz, S.A.; Im, S.-A.; Lu, Y.-S.; Colleoni, M.; Franke, F.A.; Bardia, A.; Harbeck, N.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: Overall survival (OS) results. J. Clin. Oncol. 2019, 37, LBA1008.
- Xi, J.; Oza, A.; Thomas, S.; Ademuyiwa, F.; Weilbaecher, K.; Suresh, R.; Bose, R.; Cherian, M.; Hernandez-Aya, L.; Frith, A.; et al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 141–147.
- Cook, M.; Al Rabadi, L.; Mitri, Z.I. Everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer patients previously treated with CDK4/6 inhibitor based therapies. J. Clin. Oncol. 2019, 37, 1058.
- Wander, S.A.; Zangardi, M.; Niemierko, A.; Kambadakone, A.; Kim, L.S.; Xi, J.; Pandey, A.K.; Spring, L.; Stein, C.; Juric, D.; et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, 1057.
