In recent years, there has been a growing interest for electrospun polymeric wound dressings with fiber diameters in the nano- and micrometer range. Such wound dressings display a number of properties, which support and accelerate wound healing. For instance, they provide physical and mechanical protection, exhibit a high surface area, allow gas exchange, are cytocompatible and biodegradable, resemble the structure of the native extracellular matrix, and deliver antibacterial agents locally into the wound.
- antibacterial
- antimicrobial
- biomaterial
- infection
- microfibers
- nanofibers
- scaffold
- tissue engineering
- wound dressing
1. Introduction
The wound healing process is associated with four overlapping and well-orchestrated stages: homeostasis, inflammation, proliferation and remodeling. Each stage involves a cascade of events to ensure prevention of blood loss, elimination of bacterial contamination, regeneration and formation of a new skin tissue, respectively. A variation from the norm in this process results in a delay or prolongation of any of the healing stages, which in turn leads to impaired healing [1]. The interruption in the healing process may occur for a number of reasons connected to one’s lifestyle and health condition. For example, smoking, malnutrition, obesity, low mobility, neuropathy, diabetes, vascular diseases and skin disorders have been linked to the increasing chronicity of wounds, where healing has not been achieved within 3–6 weeks [2][3][4].
Compromised wound healing represents a complex problem of multiple dependent molecular and cellular processes that are closely intertwined. A slight dysregulation in those processes leads to a development of a chronic non-healing condition, which requires a combinational approach of diverse strategies to facilitate healing. Different polymeric wound dressings have been created to supply favorable environment for wound healing, to absorb exudate, allow vapor exchange across the scaffold, maintain moist conditions, provide mechanical support and protect from further bacterial contamination. Such wound dressings have also been employed to deliver active agents such as antibiotics, antiseptics, anti-inflammatory drugs and biomolecules to direct the healing process to reach complete healing [5][6] (Figure 1).
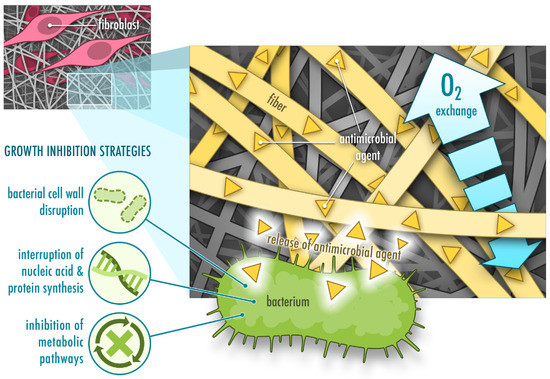
Figure 1. Functions of protein-based nanofibrous mats with incorporated antimicrobial agents for wound healing. They allow fibroblast adhesion (often through cell-recognizing motifs the fiber carries), oxygen exchange and show bacteriostatic or bactericidal activity.
The leading causes of non-healing chronic wounds are infection, pathological inflammation and formation of biofilms [2][6]. Therefore, wound care usually comprises of debridement followed by antimicrobial treatment and application of wound dressings. Debridement is required to clean the wound bed from exudate, necrotic tissue and bacterial load. Antimicrobial treatment prevents further bacterial growth and formation of biofilms. Antimicrobial agents usually follow one or several strategies to attack bacterial cells, including disruption of the bacterial cell wall, interruption of nucleic acid and protein synthesis, and dysregulation of metabolic pathways [7] (Figure 1). Antiseptics, antibiotics or other biomolecules are either applied directly or incorporated into a wound dressing [6]. In comparison to systemic administration of antimicrobial treatments, topical application requires lower concentrations, displays fewer side effects and lowers the risk of developing antibiotic resistance [6][8]. Topical application of antimicrobial agents such as antibiotics often combines a rapid initial release to kill bacteria or inhibit bacterial growth followed by a slower release to prevent further bacterial growth [9]. In order to prevent development of microbial resistance to antibiotics, silver nanoparticles have been used in certain materials for wound healing instead of antibiotics. However, recent studies demonstrate that bacterial resistance also occurs against silver nanoparticles due to an induction of nanoparticle aggregation as a result of the production of adhesive proteins by the bacteria. This problem can be overcome by additional stabilization of the nanoparticles by surfactants or polymers [10][11].
A variety of wound dressings facilitating wound healing are currently available on the market and new advanced materials are being developed (e.g., films, hydrogels, foams, hydrocolloids and nanoparticles). In particular, large research efforts have been directed to fabricate nanofibers [5][12][13]. Unlike other types of biomaterials, nanofibers stand out due to their unique structure and the tunability of their physical and mechanical properties. Their versatility and the easy fabrication process facilitate obtaining materials with desired characteristics for the complex wound healing process. High surface area and homogenous drug distribution makes nanofibers attractive as drug delivery systems with high drug loading capacity and controlled release. Resemblance of nanofibers to collagen or elastin fibers in the extracellular matrix (ECM) of healthy skin allows them to provide additional support for fibroblasts and keratinocytes, which adhere to the fibers, migrate across the wound bed and help regenerate and close the damaged tissue. Modifications of the surface morphology of nanofibers and the porosity of the nanofibrous matrix further promote adherence and migration of these cells [12] (Figure 1). However, even though electrospun fibers are often have a high porosity, this property is dependent on the fiber diameter and is difficult to control. This may also limit cell penetration into the scaffold in some cases [14].
A variety of methods to fabricate fibers have been developed over the years and mainly include solution and melt electrospinning [15]. This review focuses on nanofibers created from protein solutions using the solution electrospinning process. Electrospinning is based on applying a high voltage to a polymer solution to transform a drop at the needle tip into a cone shape in order to generate a jet. The ejected jet undergoes a number of instabilities, during which the solvent from the solution is evaporated and dry fibers are collected on the grounded or oppositely charged plate. The process is shown in Figure 2. The morphology, diameter size and distribution of electrospun fibers can be adjusted and tuned according to the solution (e.g., concentration, molecular weight, viscosity, conductivity, surface tension, dielectric constant, evaporation rate and dipole moment) and process parameters (e.g., temperature, humidity, flow rate, voltage and working distance) [16]. For example, larger fiber diameter is often associated with higher flow rate, higher applied voltage and lower distance between the needle tip and the collector. However, there are exceptions to these rules as for instance a higher voltage may lead to more solution deposition [15]. Therefore both, the properties of the solution and the process parameters should be considered during optimization of the electrospinning process [16].
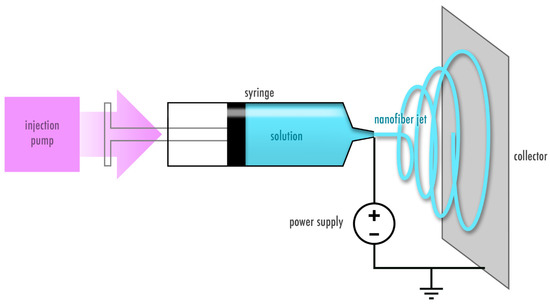
Figure 2. Electrospinning process. A polymer solution is subjected to a high voltage output to create a polymer jet that deposits as dry fibers on the collector.
The most widely used type of solution electrospinning is single-nozzle electrospinning (also known as blend electrospinning), which itself has a few subcategories with some variations including co-axial and emulsion electrospinning [15]. These techniques are commonly employed to incorporate drugs, including active biomolecules, and are summarized in Figure 3. In blend electrospinning, the drug is mixed into the polymer solution—in this case the protein solution—directly. In contrast, in co-axial electrospinning two different solutions are used, and the drug is incorporated either in the outer (shell) or inner (core) solution [17]. Additionally, the drug can be incorporated into an emulsion to be electrospun, where the final product is similar to that obtained by co-axial electrospinning due to the lengthening of the emulsion within the jet, which creates a core-shell structure [18][19]. The electrospinning technique is chosen depending on the solubility of the polymer in a particular solvent, as well as its stability during the electrospinning process and the desired release kinetics of the electrospun nanofibers. During blend electrospinning, organic and sometimes highly toxic solvents are commonly used and may affect structure, stability and activity of the drug. Therefore, co-axial and emulsion electrospinning provide an alternative, where the drug can be dissolved in a more favorable solvent [20][21][22][23]. Nevertheless, all of these techniques involve high voltage, which may potentially damage the therapeutic agent [22][23]. In such a case, there is another method that is based on a functionalization of the nanofiber surface after electrospinning by attachment of the drug (Figure 3). However, a drawback of functionalization of the fibers as compared to other methods, where the drug is incorporated into the fibers, is that the drug lacks a coating material, which normally acts as a protective layer to provide longer shelf life [21].
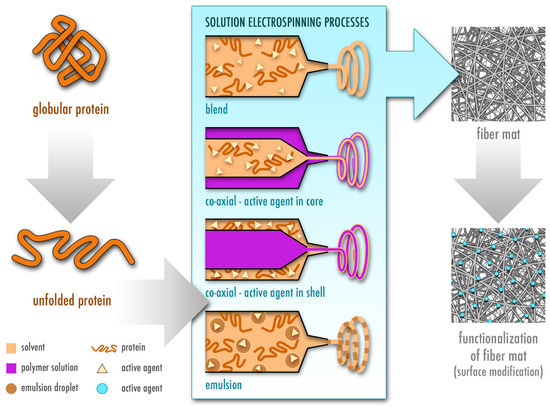
Figure 3. Fabrication of protein-based electrospun fiber mats by different types of solution electrospinning, namely blend, co-axial and emulsion electrospinning. The protein is first dissolved in a volatile solvent and starts unfolding, which is a prerequisite for successful electrospinning of proteins. In blend electrospinning, the active agent is directly added to the polymeric protein solution. In co-axial electrospinning, the active agent is either dissolved in the shell or the core solution. In addition to the protein solution, a second natural or synthetic polymer is used in co-axial electrospinning. In emulsion electrospinning, the drug is dissolved in the emulsion droplets (inner phase). In addition, the fibrous mat can be functionalized by adding the active agent after electrospinning.
2. Proteins as a Promising Starting Material for Electrospun Wound Dressings
The initial use of synthetic polymers in electrospinning has noticeably shifted towards implementation of natural polymers such as proteins and carbohydrates [24]. In comparison to synthetic polymers such as polylactic acid (PLA) [25] and polyurethane (PU) [26][27], natural polymers do not purely rely on the use of harsh and toxic organic solvents for dissolution. Therefore, they provide an environmentally friendly alternative, which may additionally offer better drug stability and activity as compared to pharmaceutical standard formulations, safer manufacturing and the possibility of an application on skin [28]. However, this comes at a cost of easy fabrication and reproducibility. Evaporation rate, surface tension and conductivity of the employed solvent greatly affect electrospinnability of the protein solution [15]. Moreover, electrospinning of proteins is more challenging due to the intrinsic variations in complexity of their structures, molecular weight, surface charge as well as ionic, hydrogen and disulfide bonds [29][30]. The electrospinnability of proteins depends not only on their solubility in a specific solvent, but also on the degree of protein unfolding in a particular solvent [29][31] and chain entanglement [31][32] (Figure 3). The choice of the solvent further affects crystallinity, mechanical properties, fiber size and morphology [29][31]. Therefore, the addition of synthetic polymers is often necessary to electrospin the solution continuously and without artifacts [24][33].
Proteins demonstrate attractive features as antimicrobial delivery system due to their natural origin, fast biodegradability and cytocompatibility [24][34]. Proteins used in electrospinning for wound healing applications are mainly obtained from two distinct sources: plants and animals [13][33]. Their stability, activity and degradation depend on the protein size, chemical structure, isolation and purification processes [5][35]. Different methods for protein extraction and purification may affect the obtained raw material’s purity and composition [5][36][37], which in turn impacts reproducibility of the electrospinning process and properties of the final product [38].
Some of the main differences between plant- and animal-based proteins are their availability and price. Plant proteins tend to be available in larger amounts and at a lower cost [31][34][39][40]. As compared to synthetic polymers, proteins are in general more challenging to electrospin due to their heterogeneity in structure and surface charge, solvent-dependent protein unfolding and low viscosity, which lead to a non-continuous electrospinning process and formation of beads [24][29]. Moreover, the final product may lack stability in water, resulting in a loss of fiber structure [41][42]. To compensate for these drawbacks, different strategies have been implemented that include the use of cross-linking agents, toxic organic solvents and addition of synthetic polymers [33].
3. Electrospinning of Plant-Derived Proteins for Wound Healing Purposes
Plant proteins that have been used to prepare electrospun wound dressings alone or in combination with other natural and/or synthetic polymers are summarized in Table 1. These include zein protein, soy protein and pea protein.
Table 1. Electrospun plant-based proteins with antimicrobial activity.
|
Protein |
Co-Polymer |
Electrospinning Type |
Solvent |
Antimicrobial Agent |
Tested Bacterial Strain |
Reference |
|
Pea |
PVA, CA |
Uniaxial |
Water |
CA |
E. coli, L. monocytogenes |
[43] |
|
Soy |
PEO |
Uniaxial |
NaOH |
None |
S. aureus, P. aeruginosa |
[44] |
|
Zein |
None |
Co-axial |
AA |
ATPPB |
E. coli, S. aureus |
[45] |
|
Zein |
PU/CA |
Uniaxial |
DMF, MEK |
Streptomycin |
E. coli, S. typhimurium, V. vulnificus, S. aureus, B. subtilis |
[26] |
|
Zein |
HA |
Uniaxial |
TFE, AA |
Salicylic acid |
S. aureus |
[46] |
|
Zein |
PU |
Uniaxial |
DMF, THF |
Ag NPs |
E. coli, S. aureus |
[27] |
|
Zein |
PCL, GA |
Uniaxial |
FA, AA |
GA |
E. coli, S. aureus |
[47] |
|
Zein |
PCL, GA |
Uniaxial, multilayer |
FA, AA |
GA, C. officinalis |
E. coli, S. aureus |
[48] |
|
Zein |
PCL |
Uniaxial |
TFE, DCM |
Tetracycline hydrochloride |
MRSA |
[49] |
|
Zein |
None |
Uniaxial |
EtOH, water |
Ag NPs |
E. coli, S. aureus |
[50] |
|
Zein |
None |
Uniaxial |
EtOH, water |
Gentamicin |
E. coli, S. aureus |
[51] |
|
Zein |
None |
Uniaxial |
EtOH, water |
Ag NPs |
E. coli, Bacillus |
[52] |
|
Zein |
None |
Co-axial |
EtOH, water |
OEO |
E. coli |
[53] |
|
Zein |
PEO |
Co-axial |
EtOH, water |
Tetracycline hydrochloride |
E. coli, S. aureus |
[54] |
|
Zein |
GT, PLA |
Uniaxial |
EtOH, water, CHL |
Tetracycline hydrochloride |
S. aureus, P. aeruginosa |
[55] |
Key: AA, acetic acid; ATPPB, allyltriphenylphosphonium bromide; CA, cinnamaldehyde; CHL, chloroform; DCM, dichloromethane; DMF, N,N-dimethylformamide; EtOH, ethanol; FA, formic acid; GA, gum arabic; GT, gum tragacanth; HA, hyaluronic acid; MEK, methyl ethyl ketone; MRSA, methicillin-resistant S. aureus; NaOH, sodium hydroxide; OEO, orange essential oil; PLA, polylactic acid; PU, polyurethane; TFE, 2,2,2-trifluoroethanol; THF, tetrahydrofuran.
4. Electrospinning Animal-Derived Proteins for Wound Healing Purposes
A lot of animal-derived proteins used in electrospinning are obtained from milk, including casein, whey, lactoferrin and lysozyme, or connective tissue, such as collagen and elastin. In comparison to plant-derived proteins that mostly require an incorporation of antimicrobial agents, proteins obtained from milk possess innate antimicrobial properties due to their iron-binding properties [56][57] and ability to disrupt bacterial cell walls [56][58]. Therefore, such proteins carry a dual function as a material basis with antimicrobial effect (Table 2).
Table 2. Electrospun animal-based proteins with antimicrobial activity.
|
Protein |
Co-Polymer |
Electrospinning Type |
Solvent |
Antimicrobial Agent |
Tested bacterial Strain |
Reference |
|
Casein |
PEO |
Uniaxial |
Water |
Ampicillin |
E. coli, S. aureus |
[59] |
|
α-lactoglobulin |
PEO |
Uniaxial |
Water |
Ampicillin |
E. coli, P. aeruginosa, B. thailandensis |
[60] |
|
Lactoferrin |
Gelatin |
Uniaxial |
FA, DMF |
None |
E. coli, S. aureus |
[61] |
|
Lysozyme |
CS, PVA |
Uniaxial |
AA, water |
CS |
S. aureus, B. subtilis, S. flexnery, P. aeruginosa |
[62] |
|
Keratin |
PVA, PEO |
Uniaxial |
NaOH |
Ag NPs |
E. coli, S. aureus |
[63] |
|
Keratin |
CS, PHBA, gelatin |
Uniaxial, multilayer |
HFIP |
Mupirocin |
E. coli, S. aureus |
[64] |
|
Collagen |
PLGA |
Uniaxial, multilayer |
HFIP |
Vancomycin hydrochloride,gentamicin sulfate |
E. coli, S. aureus |
|
|
Collagen |
PCL |
Uniaxial |
HFIP |
Enterobacteria phage T4 |
E. coli |
[68] |
|
Collagen |
PLA |
Uniaxial |
HFIP |
Levofloxacin |
E. coli, S. aureus |
[25] |
|
Collagen |
- |
Uniaxial |
HFIP |
Ag NPs |
S. aureus, P. aeruginosa |
[69] |
|
Collagen |
CS |
Uniaxial |
0.5 M AA |
ZnO |
S. aureus, E. coli |
[70] |
|
Collagen |
PCL (core), PEO (shell) |
Co-axial |
HFIP, glacial AA |
Doxycycline |
n.a. |
[71] |
|
Gelatin |
Alginate-dialde-hyde |
Uniaxial |
AA(40% w/w) |
Ciprofloxacin, gentamicin |
P. aeruginosa, S. epidermidis |
[72] |
|
Gelatin |
- |
Uniaxial |
TFE |
Vancomycin, caspofungin |
MRSA, C. albicans |
[73] |
|
Gelatin |
PMETAC |
Uniaxial |
FA, AA |
PMETAC |
S. aureus, E. coli, MRSA, A. baumannii |
[74] |
|
Gelatin |
PVA |
Uniaxial |
FA |
Centella asiatica extract |
S. aureus, E. coli, P. aeruginosa |
[75] |
|
Silk sericin |
CS |
Uniaxial |
TFA |
CS |
E. coli, B. subtilis |
[76] |
|
Silk sericin |
PLLA |
Uniaxial, multilayer |
TFA |
Nitrafurazone |
E. coli, B. subtilis |
[77] |
|
Silk sericin |
CS, PVA |
Uniaxial |
Water |
Cephalexin hydrate |
E. coli, B. subtilis |
[78] |
|
Silk fibroin |
PCL |
Uniaxial, multilayer |
HFIP |
CS |
S. aureus, E. coli |
[79] |
|
Silk fibroin |
- |
Uniaxial |
FA |
Oleuropein |
S. epidermidis, E. coli |
[80] |
|
Silk fibroin |
CS |
Uniaxial |
HFIP, TFE |
CS |
S. aureus, E. coli |
[81] |
|
Silk fibroin, sulfated fibroin |
PEI |
Uniaxial |
FA |
PEI |
S. aureus, P. aeruginosa |
[82] |
|
Silk fibroin |
- |
Uniaxial |
FA |
Ag NPs |
S. aureus, P. aeruginosa |
[83] |
|
Silk fibroin |
PVA |
Uniaxial |
Water |
EGF, ciprofloxacin hydrochloride |
S. aureus, S. epidermidis, E. coli, P. aeruginosa |
[84] |
|
Silk fibroin |
PVA |
Uniaxial |
Water |
LL-37 antimicrobial peptide, EGF |
S. epidermidis, P. aeruginosa |
[85] |
|
Silk fibroin |
Gelatin |
Uniaxial |
FA |
Thyme essential oil, doxycycline monohydrate |
S. aureus, K. pneumoniae |
[86] |
|
Melamine-modified silk fibroin |
PCL |
Uniaxial |
HFIP |
Melamine-modified silk fibroin |
S. aureus, E. coli |
[87] |
|
Silk fibroin |
PEO |
Uniaxial |
FA |
TiO2 NPs |
E. coli |
[88] |
|
Silk fibroin |
P(LLA-CL) |
Uniaxial |
HFIP |
Curcumin |
S. aureus |
[89] |
|
Silk fibroin |
PCL, HA, PEO |
Uniaxial, multilayer |
FA, TFE, water |
Thymol |
S. aureus, P. aeruginosa |
[90] |
|
Silk fibroin |
CS, halloysite nanotubes, PEO |
Uniaxial |
FA, AA, water |
Chlorhexidine digluconate |
S. aureus, E. coli |
[91] |
|
Silk fibroin |
Gelatin |
Uniaxial |
FA |
Ceftazidime |
P. aeruginosa |
[92] |
|
Silk fibroin |
|
Uniaxial |
FA |
Selenium NP coating |
S. aureus |
[93] |
|
Silk fibroin |
Carboxy-methyl CS coating |
Uniaxial |
HFIP, AA |
Carboxymethyl CS coating |
S. aureus, E. coli |
[94] |
|
Silk fibroin |
|
Uniaxial |
HFIP, FA |
Ag NP coating |
S. aureus, P. aeruginosa |
[95] |
|
Silk fibroin |
|
Uniaxial |
FA, water |
Graphene oxide coating |
S. aureus, E. coli |
[96] |
|
Silk fibroin |
PEO |
Uniaxial |
Water |
Manuka honey |
MRSA, P. aeruginosa, E. coli, S. aureus |
[97] |
|
Silk fibroin |
PEO |
Uniaxial |
Water |
Cu2O NPs |
S. aureus, E. coli |
[98] |
Key: AA, acetic acid; Ag NPs, silver nanoparticles; CS, chitosan; DMF, N,N-dimethylformamide; EGF, epidermal growth factor; FA, formic acid; HA, hyaluronic acid; HFIP, 1,1,1,3,3,3-hexafluoro-2-propanol; MRSA, methicillin-resistant S. aureus; NP, nanoparticle; PBS, phosphate buffered saline; PCL, polycaprolactone; PEI, polyethylenimine; PMETAC, poly([2-(methacryloyloxy)ethyl] trimethylammoniumchloride); PEO, polyethylene oxide; PHBA, poly(3-hydroxybutyric acid); PHBV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PLLA, poly(L-lactic acid); P(LLA-CL), poly(L-lactic acid-co-e-caprolactone); TFA, trifluoroacetic acid; TFE, 2,2,2-trifluoroethanol.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13010004
References
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.Ü.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684.
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microb. 2020, 6, 21.
- Raeder, K.; Jachan, D.E.; Muller-Werdan, U.; Lahmann, N.A. Prevalence and risk factors of chronic wounds in nursing homes in germany: A cross-sectional study. Int. Wound J. 2020, 17, 1128–1134.
- Samaniego-Ruiz, M.J.; Llatas, F.P.; Jimenez, O.S. Assessment of chronic wounds in adults: An integrative review. Rev. Esc. Enferm. USP 2018, 52, e03315.
- Ashtikar, M.; Wacker, M.G. Nanopharmaceuticals for wound healing—Lost in translation? Adv. Drug Deliv. Rev. 2018, 129, 194–218.
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56.
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49.
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549.
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003.
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71.
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in escherichia coli. Front. Genet. 2015, 6, 42.
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun nanofibrous materials for wound healing. Adv. Fiber Mater. 2020, 2, 212–227.
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67.
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305.
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. ChemPlusChem 2019, 84, 1453–1497.
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun vascular endothelial growth factor encapsulated poly(l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J. Mater. Sci. 2012, 47, 3272–3281.
- Zong, H.X.; Xia, X.; Liang, Y.R.; Dai, S.Y.; Alsaedi, A.; Hayat, T.; Kong, F.T.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091.
- Qi, H.Z.; Yang, L.J.; Shan, P.P.; Zhu, S.J.; Ding, H.; Xue, S.; Wang, Y.; Yuan, X.B.; Li, P.F. The stability maintenance of protein drugs in organic coatings based on nanogels. Pharmaceutics 2020, 12, 115.
- Puppi, D.; Chiellini, F. Drug release kinetics of electrospun fibrous systems. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 349–374.
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272.
- Tiwari, S.K.; Venkatraman, S. Electrospinning pure protein solutions in core–shell fibers. Polym. Int. 2012, 61, 1549–1555.
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 119633.
- Hall Barrientos, I.J.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337.
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892.
- Maharjan, B.; Joshi, M.K.; Tiwari, A.P.; Park, C.H.; Kim, C.S. In-situ synthesis of agnps in the natural/synthetic hybrid nanofibrous scaffolds: Fabrication, characterization and antimicrobial activities. J. Mech. Behav. Biomed. Mater. 2017, 65, 66–76.
- Zhang, B.; Yan, X.; He, H.W.; Yu, M.; Ning, X.; Long, Y.Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352.
- Aguilar-Vázquez, G.; Ortiz-Frade, L.; Figueroa-Cárdenas, J.D.; López-Rubio, A.; Mendoza, S. Electrospinnability study of pea (Pisum sativum) and common bean (Phaseolus vulgaris L.) using the conformational and rheological behavior of their protein isolates. Polym. Test. 2020, 81, 106217.
- Cho, D.; Nnadi, O.; Netravali, A.; Joo, Y.L. Electrospun hybrid soy protein/pva fibers. Macromol. Mater. Eng. 2010, 295, 763–773.
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68.
- Woerdeman, D.L.; Shenoy, S.; Breger, D. Role of chain entanglements in the electrospinning of wheat protein-poly(vinyl alcohol) blends. J. Adhes. 2007, 83, 785–798.
- Yildiz, A.; Kara, A.A.; Acarturk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097.
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-based fiber materials in medicine: A review. Nanomaterials (Basel) 2018, 8, 457.
- Jiménez, X.T.; Cuenca, A.A.; Jurado, A.T.; Corona, A.A.; Urista, C.R.M. Traditional methods for whey protein isolation and concentration: Effects on nutritional properties and biological activity. J. Mex. Chem. Soc. 2012, 56, 369–377.
- Boland, M. Whey proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 30–55.
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Chapter 22—Use of hen egg white lysozyme in the food industry. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 233–242.
- Zheng, H.; Yan, G.; Marquez, S.; Andler, S.; Dersjant-Li, Y.; de Mejia, E.G. Molecular size and immunoreactivity of ethanol extracted soybean protein concentrate in comparison with other products. Process Biochem. 2020, 96, 122–130.
- Anderson, T.J.; Lamsal, B.P. Review: Zein extraction from corn, corn products, and coproducts and modifications for various applications: A review. Cereal Chem. 2011, 88, 159–173.
- Yeo, G.C.; Aghaei-Ghareh-Bolagh, B.; Brackenreg, E.P.; Hiob, M.A.; Lee, P.; Weiss, A.S. Fabricated elastin. Adv. Health. Mater. 2018, 7, e1801342.
- Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. Investigation and prevention of clogging during electrospinning of zein solution. J. Appl. Polym. Sci. 2010, 118, 1821–1829.
- Vogt, L.; Liverani, L.; Roether, J.A.; Boccaccini, A.R. Electrospun zein fibers incorporating poly(glycerol sebacate) for soft tissue engineering. Nanomaterials 2018, 8, 150.
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 393–402.
- Thirugnanaselvam, M.; Gobi, N.; Arun Karthick, S. Spi/peo blended electrospun martrix for wound healing. Fibers Polym. 2013, 14, 965–969.
- Li, J.J.; Feng, H.T.; He, J.M.; Li, C.; Mao, X.; Xie, D.M.; Ao, N.J.; Chu, B. Coaxial electrospun zein nanofibrous membrane for sustained release. J. Biomater. Sci. Polym. Ed. 2013, 24, 1923–1934.
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110.
- Pedram, R.Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of pcl/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366.
- Pedram, R.Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/pcl/zein/gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543.
- Alhusein, N.; Blagbrough, I.S.; Beeton, M.L.; Bolhuis, A.; De Bank, P.A. Electrospun zein/pcl fibrous matrices release tetracycline in a controlled manner, killing staphylococcus aureus both in biofilms and ex vivo on pig skin, and are compatible with human skin cells. Pharm. Res. 2016, 33, 237–246.
- Dashdorj, U.; Reyes, M.K.; Unnithan, A.R.; Tiwari, A.P.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Fabrication and characterization of electrospun zein/ag nanocomposite mats for wound dressing applications. Int. J. Biol. Macromol. 2015, 80, 1–7.
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2057–2070.
- Ullah, S.; Hashmi, M.; Khan, M.Q.; Kharaghani, D.; Saito, Y.; Yamamoto, T.; Kim, I.S. Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv. 2019, 9, 268–277.
- Yao, Z.C.; Chen, S.C.; Ahmad, Z.; Huang, J.; Chang, M.W.; Li, J.S. Essential oil bioactive fibrous membranes prepared via coaxial electrospinning. J. Food Sci. 2017, 82, 1412–1422.
- Akhmetova, A.; Lanno, G.-M.; Kogermann, K.; Malmsten, M.; Rades, T.; Heinz, A. Highly elastic and water stable zein microfibers as a potential drug delivery system for wound healing. Pharmaceutics 2020, 12, 458.
- Ghorbani, M.; Mahmoodzadeh, F.; Yavari Maroufi, L.; Nezhad-Mokhtari, P. Electrospun tetracycline hydrochloride loaded zein/gum tragacanth/poly lactic acid nanofibers for biomedical application. Int. J. Biol. Macromol. 2020, 165, 1312–1322.
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091.
- Oram, J.D.; Reiter, B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta Gen. Subj. 1968, 170, 351–365.
- Sarkar, S.; Gulati, K.; Mishra, A.; Poluri, K.M. Protein nanocomposites: Special inferences to lysozyme based nanomaterials. Int. J. Biol. Macromol. 2020, 151, 467–482.
- Selvaraj, S.; Thangam, R.; Fathima, N.N. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int. J. Biol. Macrom. 2018, 120, 1674–1681.
- Stie, M.B.; Corezzi, M.; Juncos Bombin, A.D.; Ajalloueian, F.; Attrill, E.; Pagliara, S.; Jacobsen, J.; Chronakis, I.S.; Nielsen, H.M.; Foderà, V. Waterborne electrospinning of α-lactalbumin generates tunable and biocompatible nanofibers for drug delivery. ACS Appl. Nano Mater. 2020, 3, 1910–1921.
- Padrão, J.; Machado, R.; Casal, M.; Lanceros-Méndez, S.; Rodrigues, L.R.; Dourado, F.; Sencadas, V. Antibacterial performance of bovine lactoferrin-fish gelatine electrospun membranes. Int. J. Biol. Macromol. 2015, 81, 608–614.
- Park, J.M.; Kim, M.; Park, H.S.; Jang, A.; Min, J.; Kim, Y.H. Immobilization of lysozyme-clea onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43.
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun silver nanoparticles-embedded feather keratin/poly(vinyl alcohol)/poly(ethylene oxide) antibacterial composite nanofibers. Polymers 2020, 12, 305.
- Singaravelu, S.; Ramanathan, G.; Muthukumar, T.; Raja, M.D.; Nagiah, N.; Thyagarajan, S.; Aravinthan, A.; Gunasekaran, P.; Natarajan, T.S.; Selva, G.V.; et al. Durable keratin-based bilayered electrospun mats for wound closure. J. Mater. Chem. B 2016, 4, 3982–3997.
- Chen, D.W.; Hsu, Y.H.; Liao, J.Y.; Liu, S.J.; Chen, J.K.; Ueng, S.W.N. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured plga/collagen nanofibrous membranes. Int. J. Pharmaceut. 2012, 430, 335–341.
- Chen, D.W.; Lee, F.Y.; Liao, J.Y.; Liu, S.J.; Hsiao, C.Y.; Chen, J.K. Preclinical experiments on the release behavior of biodegradable nanofibrous multipharmaceutical membranes in a model of four-wall intrabony defect. Antimicrob. Agents Chemother. 2013, 57, 9–14.
- Chen, D.W.; Liao, J.Y.; Liu, S.J.; Chan, E.C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 2012, 7, 763–771.
- Cheng, W.; Zhang, Z.; Xu, R.; Cai, P.; Kristensen, P.; Chen, M.; Huang, Y. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2588–2595.
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J. Drug Target. 2016, 24, 520–529.
- Sun, L.; Han, J.; Liu, Z.; Wei, S.; Su, X.; Zhang, G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated collagenous chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: Exhaustive in vivo evaluations. J. Photochem. Photobiol. B 2019, 197, 111539.
- Tort, S.; Acarturk, F.; Besikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653.
- Chen, J.; Liu, Z.; Chen, M.; Zhang, H.; Li, X. Electrospun gelatin fibers with a multiple release of antibiotics accelerate dermal regeneration in infected deep burns. Macromol. Biosci. 2016, 16, 1368–1380.
- Dhand, C.; Barathi, V.A.; Ong, S.T.; Venkatesh, M.; Harini, S.; Dwivedi, N.; Goh, E.T.; Nandhakumar, M.; Venugopal, J.R.; Diaz, S.M.; et al. Latent oxidative polymerization of catecholamines as potential cross-linkers for biocompatible and multifunctional biopolymer scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 32266–32281.
- Inal, M.; Mulazimoglu, G. Production and characterization of bactericidal wound dressing material based on gelatin nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404.
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915.
- Zhao, R.; Li, X.; Sun, B.L.; Zhang, Y.; Zhang, D.W.; Tang, Z.H.; Chen, X.S.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97.
- Zhao, R.; Li, X.; Sun, B.L.; Tong, Y.; Jiang, Z.Q.; Wang, C. Nitrofurazone-loaded electrospun plla/sericin-based dual-layer fiber mats for wound dressing applications. RSC Adv. 2015, 5, 16940–16949.
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Prospects of nonmulberry silk protein sericin-based nanofibrous matrices for wound healing - in vitro and in vivo investigations. Acta Biomater. 2018, 78, 137–150.
- Wu, G.M.; Ma, X.; Fan, L.; Gao, Y.Y.; Deng, H.B.; Wang, Y.N. Accelerating dermal wound healing and mitigating excessive scar formation using lbl modified nanofibrous mats. Mater. Des. 2020, 185, 108265.
- Bayraktar, O.; Balta, A.B.; Bayraktar, G.B. Adsorption/desorption and biofunctional properties of oleuropein loaded on different types of silk fibroin matrices. Maced. J. Chem. Chem. Eng. 2017, 36, 153–165.
- Cai, Z.X.; Mo, X.M.; Zhang, K.H.; Fan, L.P.; Yin, A.L.; He, C.L.; Wang, H.S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539.
- Calamak, S.; Erdogdu, C.; Ozalp, M.; Ulubayram, K. Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mat. Sci. Eng. C Mater. 2014, 43, 11–20.
- Calamak, S.; Aksoy, E.A.; Erdogdu, C.; Sagiroglu, M.; Ulubayram, K. Silver nanoparticle containing silk fibroin bionanotextiles. J. Nanopart. Res. 2015, 17, 87.
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174.
- Chouhan, D.; Janani, G.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Functionalized pva-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J. Tissue Eng. Regen. M. 2018, 12, E1559–E1570.
- Chomachayi, M.D.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. A 2018, 106, 1092–1103.
- Hu, W.K.; Wang, Z.J.; Xu, Y.; Wang, X.H.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Remodeling of inherent antimicrobial nanofiber dressings with melamine-modified fibroin into neoskin. J. Mater. Chem. B 2019, 7, 3412–3423.
- Jao, W.C.; Yang, M.C.; Lin, C.H.; Hsu, C.C. Fabrication and characterization of electrospun silk fibroin/tio2 nanofibrous mats for wound dressings. Polym. Adv. Technol. 2012, 23, 1066–1076.
- Lian, Y.; Zhan, J.C.; Zhang, K.H.; Mo, X.M. Fabrication and characterization of curcumin-loaded silk fibroin/p(lla-cl) nanofibrous scaffold. Front. Mater. Sci. 2014, 8, 354–362.
- Miguel, S.P.; Simoes, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535.
- Ren, X.X.; Xu, Z.P.; Wang, L.B.; Meng, K.; Wang, H.; Zhao, H.J. Silk fibroin/chitosan/halloysite composite medical dressing with antibacterial and rapid haemostatic properties. Mater. Res. Express 2019, 6, 125409.
- Safdari, M.; Shakiba, E.; Kiaie, S.H.; Fattahi, A. Preparation and characterization of ceftazidime loaded electrospun silk fibroin/gelatin mat for wound dressing. Fiber Polym. 2016, 17, 744–750.
- Chung, S.; Ercan, B.; Roy, A.K.; Webster, T.J. Addition of selenium nanoparticles to electrospun silk scaffold improves the mammalian cell activity while reducing bacterial growth. Front. Physiol. 2016, 7, 297.
- Tu, H.; Wu, G.M.; Yi, Y.; Huang, M.T.; Liu, R.; Shi, X.W.; Deng, H.B. Layer-by-layer immobilization of amphoteric carboxymethyl chitosan onto biocompatible silk fibroin nanofibrous mats. Carbohydr. Polym. 2019, 210, 9–16.
- Uttayarat, P.; Jetawattana, S.; Suwanmala, P.; Eamsiri, J.; Tangthong, T.; Pongpat, S. Antimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing application. Fiber Polym. 2012, 13, 999–1006.
- Wang, S.D.; Ma, Q.; Wang, K.; Chen, H.W. Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. ACS Omega 2018, 3, 406–413.
- Yang, X.X.; Fan, L.P.; Ma, L.L.; Wang, Y.Y.; Lin, S.; Yu, F.; Pan, X.H.; Luo, G.J.; Zhang, D.D.; Wang, H.S. Green electrospun manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84.
- Zheng, Z.X.; Zhang, K.H.; Wu, B.; Yang, H.Y.; Wang, M.Q.; Dong, T.H.; Zhang, J.Y.; He, Y. Green electrospun nanocuprous oxide-poly(ethylene oxide)-silk fibroin composite nanofibrous scaffolds for antibacterial dressings. J. Appl. Polym. Sci. 2019, 136, 47730.
