The hippocampus is crucial in learning, memory and emotion processing, and is involved in the development of different neurological and neuropsychological disorders. Several epigenetic factors, including DNA methylation, histone modifications and non-coding RNAs, have been shown to regulate the development and function of the hippocampus, and the alteration of epigenetic regulation may play important roles in the development of neurocognitive and neurodegenerative diseases.
- epigenetics
- hippocampus
- radiation
- histones
- neurogenesis
- miRNA
- DNA methylation
1. Introduction
The hippocampus is a structure in the brain within the medial temporal lobe [1] which plays an important role in the limbic system and is involved in learning, memory processing and emotions [2]. The three principle structures of the hippocampal formation (hippocampus, dentate gyrus and subiculum) are made up of glutamatergic principal neurons and inhibitory GABAergic neurons [3], and damage to the hippocampus has been shown to bring about amnesic effects in humans, alluding to its major role in memory formation and consolidation [4]. Both animal and clinical studies have clearly indicated that radiation exposure or radiotherapy induces hippocampal damage, resulting in the impairment of neurogenesis and cognition [5]. The production of reactive oxygen species (ROS), pro-inflammatory cytokines and chemokines, pro-apoptotic proteins, autophagosome markers, excitatory neurotransmitters and neurotrophic factors may be involved in hippocampal neuropathy and cognitive impairment [6][7]. Recent studies have suggested that epigenetic mechanisms are involved not only in normal hippocampal development and regulation [8][9][10][11][12][13][14], but also in radiation-induced hippocampal pathophysiological changes leading to the development of neurological and neuropsychological disorders [15].
2. Epigenetic Regulation of Hippocampal Neurogenesis
The generation of new neurons in the adult central nervous system (CNS) in the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus is one of the major breakthroughs in neuroscience research. Adult hippocampal neurogenesis occurs in the DG and refers to the formation of new functional dentate granule cells from neural stem cells (NSCs) [10] contributing to learning and memory [11] and also mood regulation [16]. Adult hippocampal neurogenesis was first observed in rodents [17] and later confirmed in humans [18][19][20]. This process occurs when intermediate neural progenitors (IPCs) are amplified and integrated into existing neural circuits. Due to this, adult hippocampal neurogenesis provides a means for both functional and structural plasticity in the hippocampus. Dysregulation of adult hippocampal neurogenesis has been shown to cause cognitive decline and psychological symptoms [10].
The process of adult hippocampal neurogenesis is regulated by factors that are both extrinsic and intrinsic [21], which are able to actively upregulate or downregulate the generation of new neurons throughout adulthood and may occur prenatally or postnatally. One of the key regulators of neurogenesis is epigenetics. Studies have shown that epigenetic regulators are crucial for the generation of neurons from adult neural progenitors that integrate into the hippocampus [8][22][23][24]. These neural progenitor cells have high levels of histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 27 trimethylation (H3K27me3); alterations of H3K4me3 and H3K27me3 are often the focus of environmental epigenetic studies due to their strong association with gene expression at promoters. Histone deacetylase 1 (HDAC1) is mainly expressed in NSCs, while mature neurons mainly express histone deacetylase 2 (HDAC2) [25] suggesting that the expression of HDACs may be developmentally regulated. Combined deletion of both these HDACs resulted in an inability for neuronal precursors to differentiate into mature neurons, leading to excessive cell death [26]. In cases where HDAC2 was depleted, studies have shown that HDAC1 was able to compensate for the loss of HDAC2 [27], and thus, neurogenesis is not affected. In terms of histone acetylation, KAT6B, a gene which provides instructions for making histone acetyltransferases, is highly expressed in the adult SVZ, and a deficiency of KAT6B results in a decrease in NSCs [28].
Tritorax group (trxG) and polycomb group (PcG) proteins activate or silence gene expression respectively, through a chromatin remodeling system [29]. Epigenetic modifications of chromatin structure by PcG proteins, which function as transcriptional repressors, aid in the maintenance of cellular identity [30]. These proteins facilitate the trimethylation of the lysine 27 of histone 3 (H3K27me3), bringing about transcriptional repression, and trxG protein complexes catalyze the trimethylation of H3K4 (H3K4me3) [11]. Mixed-lineage leukemia I (Mll1) is a member of the trxG family and it is expressed in the SVZ and olfactory bulb (OB) [31]. Mll1 has been shown to be crucial for the proliferation and neurogenesis of SVZ and the olfactory bulb (OB) NSCs [29] and is known to be a histone methyltransferase (HMT) for histone H3 lysine 4 [32]. Studies have shown that a deficiency of Mll1 in the SVZ severely impaired neuronal differentiation [29].
Sex determining region Y-box 2 (SOX2) has been reported to prime the epigenetic landscape in neural progenitors, as the early SOX2-dependent imbalance in H3K4me3 and H3K27me3 marks has a profound impact throughout the entire differentiation process, which allows for proper gene activation during neurogenesis [8]. The epigenetic mechanisms that regulate neurogenesis are extremely strongly associated with each other and other regulatory pathways [33].
The involvement of DNA methylation is seen in studies with Gadd45b, which is a crucial component for the DNA methylation of certain promoters and their corresponding gene expressions essential for neurogenesis [23], thereby increasing the expression of key neuronal genes such as Fgf1 and Bdnf [28]. Mice with Gadd45b deletions showed deficits in the proliferation of neural progenitors and dendritic growth in the hippocampus [23]. In addition, mice exposed to prenatal stress showed increased expressions of DNA methyltransferase 1 (DNMT1), and also an increase in its binding to the glutamic acid decarboxylase 67 (GAD67) promoter, leading to an impairment in the genesis of the gamma-aminobutyric acid (GABA) interneurons, suggesting that prenatal upregulation of DNMT1 may reduce interneuron genesis [10]. On the other hand, the expression of DMNT1 in neural precursor cells was also shown to be crucial for the survival of newly generated neurons in the adult hippocampus. Deletion of DNMT1 in NSCs at an early stage of DG development impaired the ability of NSCs to establish secondary radial glial scaffolds and to migrate into the SGZ of the DG, leading to aberrant neuronal production in the molecular layer, increased cell death and decreased granule neuron production. Furthermore, it promoted the differentiation of NSCs into astrocytes [34][35]. In addition to DMNT1, DMNT3-knockout mice were also found to have significantly fewer immature neurons [36]. DNMT1 has been shown to control the timing of astrogliogenesis through Janus kinase/signal transducers and activators of transcription (JAK-STAT) signaling, and DNMT3A and DNMT3B are required for neuron specification [28]. Maternal exposure to 3,3’-iminodipropionitrile (IDPN) was shown to affect hippocampal neurogenesis in offspring, and this was found to be due to hypermethylation of genes Edc4, Kiss1 and Mrpl38 [13]. DMNTs have been proven to be crucial in ensuring normal neurogenesis, as dysregulation or mutations in DNMTs result in abnormal neurogenesis [37] which further confirms the importance of DNA methylation in hippocampal neurogenesis. Reductions in hippocampal neurogenesis have also been linked to glucocorticoid hormones (GC) which regulate neural stem/precursor cell proliferation via changes in the methylation state of gene promoters associated with cell cycle regulation and Wnt signaling [38]. Disruption of GCs causes alterations in dendritic morphology and numbers and the appearance of new granule neurons.
Methyl-CpG-binding protein 2 (MeCP2) is a member of the methyl-CpG binding domain (MBD) family that plays an important role in both neuronal and astrocytic lineage specification by repressing astrocytic genes during neurogenesis and releasing this repression during astrogenesis [39]. MeCP2 appears to play a key role in conveying neuronal signaling and activity into epigenetic gene regulation. MeCP2-knockout mice have neurons with smaller nuclei and fewer dendritic branches [40], and activity-dependent changes in DNA methylation were associated with a reduction in MeCP2 binding [41]. MeCp2 deficiency also correlated with poor neural progenitor cell (NPC) maturation and impaired dendritic and spine morphogenesis in new neurons [42]. In addition to MeCP2, MBD1 proteins of the MBD family have also been shown to play crucial roles in hippocampal neurogenesis [33], and it is their MBD domains that interact with methylated CpG, and thus, MBD binding correlates with DNA methylation. MBD1-deficient mice had lower levels of neurogenesis and impaired spatial learning capabilities [43] and were also found to be susceptible to depression [44]. It is evident that DNA methylation has a crucial role in the maintenance of NPCs and their fate specification in adult hippocampal neurogenesis.
It has also been shown that MeCP2 is able to epigenetically regulate miRNAs in adult NSCs, which brings in another form of epigenetic regulation. Co-regulation of miR-137 together with SOX2 regulates the proliferation and differentiation of adult neural stem cells [45]. Knockdown of miR-137 enhances their differentiation while overexpression of miR-137 promotes their proliferation. Furthermore, miR-137 has been found to repress the expression of enhancer of zeste homolog 2 (EZH2), a PcG histone methytransferase, which brings about a global reduction in H3K27me3-modulated neurogenesis [45]. Similarly, high levels of miR-184 were found to promote proliferation of neural progenitors while inhibiting their differentiation [46].
The miR-30 family of miRNAs has also been shown to mediate the effects of stress on hippocampal neurogenesis in mice [47]. These miRNAs were found to be downregulated in stressed mice and involved in neural progenitor cells differentiation. In addition, miR-137 and miR-34a have been shown to negatively regulate dendritic branching and the complexity of newborn neurons [48][49], and miR-19 has been proven to be crucial for the migration of these newborn neurons [50]. Human neural progenitor cells with abnormal expression of miR-19 display deviant migration patterns. Regulation of ten-eleven translocation protein 1 (TET1), a methylcytosine dioxygenase, and miR-124 has also been shown to modulate hippocampal neurogenesis. Together with miR-9, miR-124 appears to repress Brg- and Brahma (Brm)- associated factor-complex 53a (BAF53a) allowing neural progenitors to properly differentiate into neurons [32]. Thus, miR-124 is very lowly expressed in progenitor cells but upregulated in differentiation and mature neurons [51]. TET1 controls the demethylation of miR-124 thereby regulating its expression. Dysregulation of TET1 and miR-124 due to Down Syndrome critical region 1 (DSCR1) protein knockout led to an impairment in hippocampal neurogenesis [52]. TET1 is also known to interact with MeCP2 further confirming the importance of epigenetic regulation including the crosstalk between different epigenetic factors [53]. Several studies also show that a synergistic modulation by various different miRNAs facilitates hippocampal neurogenesis and is crucial for neurogenic lineage fate determination in the adult hippocampus [54][55][56]. Epigenetic factors governing adult hippocampal neurogenesis are summarized in Figure 1. We are just beginning to understand the influence of epigenetics on hippocampal neurogenesis as an intermediate regulatory mechanism between DNA sequences and gene expression. Thus, further studies should be carried out on the molecular pathways to induce, remove and interpret epigenetic modifications. Looking downstream, this may enable us to further elucidate the mechanisms of neurodevelopment-related disorders and aid the development of novel therapeutic approaches to prevent abnormal brain development.
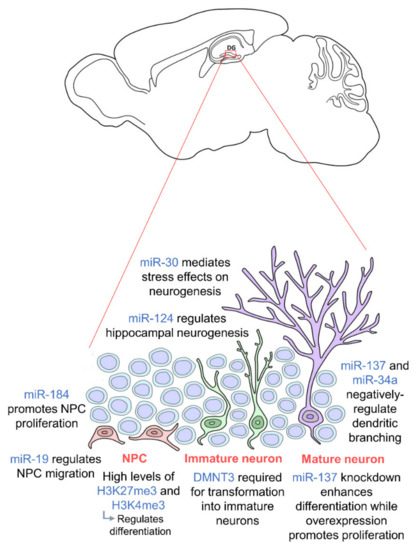
Figure 1. Epigenetic regulation during adult hippocampal neurogenesis. Adult hippocampal neurogenesis is regulated by epigenetics at every stage. Neural progenitor cells (NPCs) (orange) require high levels of H3K27me3 and H3K4me3 for proper differentiation, and their proliferation and migration are regulated by miR-184 and miR-19 respectively. In order for NPCs to transform into immature neurons (green), DMNT3 is required. Mature neurons (purple) are then regulated by miR-137 and miR-34a. miR-30 and miR-124 regulate adult hippocampal neurogenesis in general. NPCs are located in the subgranular zone (SGZ) of the dentate gyrus, and mature neurons integrate into the granule cell layer. (DNMT: DNA methyltransferase).
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249514
References
- Knierim, J.J. The hippocampus. Current Biology 2015, 25, R1116-R1121.
- Tatu, L.; Vuillier, F. Structure and vascularization of the human hippocampus. In The Hippocampus in Clinical Neuroscience, Karger Publishers: 2014; Vol. 34, pp. 18-25.
- Fink, G. Stress: Physiology, Biochemistry, and Pathology: Handbook of Stress Series; Academic Press: 2019; Vol. 3.
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harbor perspectives in biology 2015, 7, a021766.
- Bledsoe, J.C. Effects of cranial radiation on structural and functional brain development in pediatric brain tumors. Journal of Pediatric Neuropsychology 2016, 2, 3-13.
- Yang, B.; Ren, B.X.; Tang, F.R. Prenatal irradiation–induced brain neuropathology and cognitive impairment. Brain and Development 2017, 39, 10-22.
- Tang, F.R.; Loke, W.K.; Khoo, B.C. Low-dose or low-dose-rate ionizing radiation–induced bioeffects in animal models. Journal of radiation research 2017, 58, 165-182.
- Amador-Arjona, A.; Cimadamore, F.; Huang, C.-T.; Wright, R.; Lewis, S.; Gage, F.H.; Terskikh, A.V. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proceedings of the National Academy of Sciences 2015, 112, E1936-E1945.
- Mews, P.; Donahue, G.; Drake, A.M.; Luczak, V.; Abel, T.; Berger, S.L. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 2017, 546, 381-386.
- Zhong, H.; Rong, J.; Zhu, C.; Liang, M.; Li, Y.; Zhou, R. Epigenetic modifications of GABAergic interneurons contributes to the deficits in adult hippocampus neurogenesis and depression-like behavior in prenatally stressed mice. International Journal of Neuropsychopharmacology 2020.
- Covic, M.; Karaca, E.; Lie, D. Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity 2010, 105, 122-134.
- Watanabe, Y.; Abe, H.; Nakajima, K.; Ideta-Otsuka, M.; Igarashi, K.; Woo, G.-H.; Yoshida, T.; Shibutani, M. Aberrant epigenetic gene regulation in GABAergic interneuron subpopulations in the hippocampal dentate gyrus of mouse offspring following developmental exposure to hexachlorophene. Toxicological Sciences 2018, 163, 13-25.
- Tanaka, T.; Nakajima, K.; Masubuchi, Y.; Ito, Y.; Kikuchi, S.; Ideta-Ohtsuka, M.; Woo, G.-H.; Yoshida, T.; Igarashi, K.; Shibutani, M. Aberrant epigenetic gene regulation in hippocampal neurogenesis of mouse offspring following maternal exposure to 3, 3’-iminodipropionitrile. The Journal of toxicological sciences 2019, 44, 93-105.
- Fan, S.J.; Sun, A.B.; Liu, L. Epigenetic modulation during hippocampal development. Biomedical reports 2018, 9, 463-473.
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxidative medicine and cellular longevity 2019, 2019.
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645-660.
- Altman, J. Are new neurons formed in the brains of adult mammals? Science 1962, 135, 1127-1128.
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nature medicine 1998, 4, 1313-1317.
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PloS one 2010, 5.
- Roy, N.S.; Wang, S.; Jiang, L.; Kang, J.; Benraiss, A.; Harrison-Restelli, C.; Fraser, R.A.; Couldwell, W.T.; Kawaguchi, A.; Okano, H. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nature medicine 2000, 6, 271-277.
- Balu, D.T.; Lucki, I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neuroscience & Biobehavioral Reviews 2009, 33, 232-252.
- Sun, J.; Sun, J.; Ming, G.l.; Song, H. Epigenetic regulation of neurogenesis in the adult mammalian brain. European Journal of Neuroscience 2011, 33, 1087-1093.
- Ma, D.K.; Jang, M.-H.; Guo, J.U.; Kitabatake, Y.; Chang, M.-l.; Pow-Anpongkul, N.; Flavell, R.A.; Lu, B.; Ming, G.-l.; Song, H. Neuronal activity–induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009, 323, 1074-1077.
- Gao, Z.; Ure, K.; Ding, P.; Nashaat, M.; Yuan, L.; Ma, J.; Hammer, R.E.; Hsieh, J. The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. Journal of Neuroscience 2011, 31, 9772-9786.
- MacDonald, J.L.; Roskams, A.J. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro‐glial development. Developmental dynamics: an official publication of the American Association of Anatomists 2008, 237, 2256-2267.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death & Differentiation 2018, 25, 486-541.
- Montgomery, R.L.; Hsieh, J.; Barbosa, A.C.; Richardson, J.A.; Olson, E.N. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proceedings of the National Academy of Sciences 2009, 106, 7876-7881.
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. International Journal of Molecular Sciences 2020, 21, 4869.
- Lim, D.A.; Huang, Y.-C.; Swigut, T.; Mirick, A.L.; Garcia-Verdugo, J.M.; Wysocka, J.; Ernst, P.; Alvarez-Buylla, A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature 2009, 458, 529-533.
- Boyer, L.A.; Plath, K.; Zeitlinger, J.; Brambrink, T.; Medeiros, L.A.; Lee, T.I.; Levine, S.S.; Wernig, M.; Tajonar, A.; Ray, M.K. Polycomb complexes repress developmental regulators in murine embryonic stem cells. nature 2006, 441, 349-353.
- Lim, D.A.; Alvarez-Buylla, A. The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harbor perspectives in biology 2016, 8, a018820.
- Hsieh, J.; Eisch, A.J. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiology of disease 2010, 39, 73-84.
- Jobe, E.M.; Zhao, X. DNA methylation and adult neurogenesis. Brain Plasticity 2017, 3, 5-26.
- Noguchi, H.; Kimura, A.; Murao, N.; Matsuda, T.; Namihira, M.; Nakashima, K. Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neuroscience research 2015, 95, 1-11.
- Noguchi, H.; Murao, N.; Kimura, A.; Matsuda, T.; Namihira, M.; Nakashima, K. DNA methyltransferase 1 is indispensable for development of the hippocampal dentate gyrus. Journal of Neuroscience 2016, 36, 6050-6068.
- Wu, H.; Coskun, V.; Tao, J.; Xie, W.; Ge, W.; Yoshikawa, K.; Li, E.; Zhang, Y.; Sun, Y.E. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 2010, 329, 444-448.
- Mohan, K.N. Stem cell models to investigate the role of DNA methylation machinery in development of neuropsychiatric disorders. Stem cells international 2016, 2016.
- Schouten, M.; Bielefeld, P.; Garcia-Corzo, L.; Passchier, E.; Gradari, S.; Jungenitz, T.; Pons-Espinal, M.; Gebara, E.; Martín-Suárez, S.; Lucassen, P.J. Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Molecular psychiatry 2019, 1-24.
- Namihira, M.; Nakashima, K.; Taga, T. Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS letters 2004, 572, 184-188.
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.-n.; Ho, H.-y.H.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52, 255-269.
- Martinowich, K.; Hattori, D.; Wu, H.; Fouse, S.; He, F.; Hu, Y.; Fan, G.; Sun, Y.E. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003, 302, 890-893.
- Smrt, R.D.; Eaves-Egenes, J.; Barkho, B.Z.; Santistevan, N.J.; Zhao, C.; Aimone, J.B.; Gage, F.H.; Zhao, X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiology of disease 2007, 27, 77-89.
- Zhao, X.; Ueba, T.; Christie, B.R.; Barkho, B.; McConnell, M.J.; Nakashima, K.; Lein, E.S.; Eadie, B.D.; Willhoite, A.R.; Muotri, A.R. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proceedings of the National Academy of Sciences 2003, 100, 6777-6782.
- Allan, A.M.; Liang, X.; Luo, Y.; Pak, C.; Li, X.; Szulwach, K.E.; Chen, D.; Jin, P.; Zhao, X. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Human molecular genetics 2008, 17, 2047-2057.
- Szulwach, K.E.; Li, X.; Smrt, R.D.; Li, Y.; Luo, Y.; Lin, L.; Santistevan, N.J.; Li, W.; Zhao, X.; Jin, P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. Journal of Cell Biology 2010, 189, 127-141.
- Liu, C.; Teng, Z.-Q.; Santistevan, N.J.; Szulwach, K.E.; Guo, W.; Jin, P.; Zhao, X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell stem cell 2010, 6, 433-444.
- Khandelwal, N.; Dey, S.K.; Chakravarty, S.; Kumar, A. miR-30 family miRNAs mediate the effect of chronic social defeat stress on hippocampal neurogenesis in mouse depression model. Frontiers in molecular neuroscience 2019, 12, 188.
- Antonini, D.; Russo, M.T.; De Rosa, L.; Gorrese, M.; Del Vecchio, L.; Missero, C. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. Journal of Investigative Dermatology 2010, 130, 1249-1257.
- Smrt, R.D.; Szulwach, K.E.; Pfeiffer, R.L.; Li, X.; Guo, W.; Pathania, M.; Teng, Z.Q.; Luo, Y.; Peng, J.; Bordey, A. MicroRNA miR‐137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb‐1. Stem cells 2010, 28, 1060-1070.
- Han, J.; Kim, H.J.; Schafer, S.T.; Paquola, A.; Clemenson, G.D.; Toda, T.; Oh, J.; Pankonin, A.R.; Lee, B.S.; Johnston, S.T. Functional implications of miR-19 in the migration of newborn neurons in the adult brain. Neuron 2016, 91, 79-89.
- Deo, M.; Yu, J.Y.; Chung, K.H.; Tippens, M.; Turner, D.L. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Developmental dynamics: an official publication of the American Association of Anatomists 2006, 235, 2538-2548.
- Choi, C.; Kim, T.; Chang, K.T.; Min, K.T. DSCR1‐mediated TET1 splicing regulates miR‐124 expression to control adult hippocampal neurogenesis. The EMBO journal 2019, 38.
- Zheng, Z.; Ambigapathy, G.; Keifer, J. MeCP2 regulates Tet1-catalyzed demethylation, CTCF binding, and learning-dependent alternative splicing of the BDNF gene in Turtle. Elife 2017, 6, e25384.
- Pons-Espinal, M.; de Luca, E.; Marzi, M.J.; Beckervordersandforth, R.; Armirotti, A.; Nicassio, F.; Fabel, K.; Kempermann, G.; Tonelli, D.D.P. Synergic functions of miRNAs determine neuronal fate of adult neural stem cells. Stem cell reports 2017, 8, 1046-1061.
- Bielefeld, P.; Mooney, C.; Henshall, D.C.; Fitzsimons, C.P. miRNA-mediated regulation of adult hippocampal neurogenesis; implications for epilepsy. Brain Plasticity 2017, 3, 43-59.
- Stappert, L.; Klaus, F.; Brüstle, O. MicroRNAs engage in complex circuits regulating adult neurogenesis. Frontiers in neuroscience 2018, 12, 707.
