Discussion
In the past few decades, platelet concentrate has emerged as an adjunct therapeutic for musculoskeletal injury. The rationale for its use is largely dependent on its functional components, which include a variety of growth factors, coagulation factors, adhesion molecules, cytokines, and chemokines [
14,
25,
26,
27,
28]. Upon activation, the platelets can release these anabolic growth factors at concentrations significantly higher than the baseline blood levels [
27].
The aims of this present study are to demonstrate the utility and capacity of PRF to serve as a “cytokine-delivery vehicle” in promoting Achilles tendon healing. In our previous studies, PRF demonstrated pronounced effects on cellular migration, viability, and differentiation both in vitro and in vivo, which may translate into a viable strategy in promoting tendinous healing. Nonetheless, the cytokine-release kinetics of PRF and its influence on tendinous healing remained largely unknown. Based on our results, the essence portion of PRF was successfully defined and its bioactivity on cultured tenocytes were revealed. When implanted in vivo, PRF can improve functional tendinous healing evidenced by imaging and histological results. From a clinical standpoint, our data demonstrate a new and effective way to improve tendinous healing.
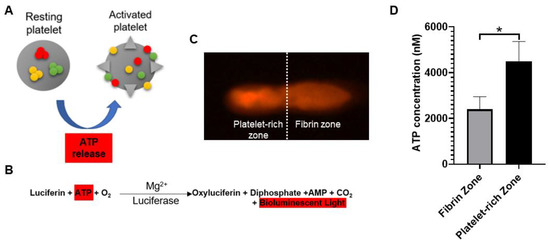
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate produced from autologous blood obtained immediately after single-spin centrifugation [
14]. Rapid activation of the coagulation cascade and synthesis of thrombin take place when the platelets come into contact with the glass particles of the test tube. The ATP is co-packaged in platelet–dense granules with serotonin, Ca
2+, and ADP and are secreted when the platelets are activated [
24,
29]. In the current study, the distribution of activated platelets on the PRF scaffold was identified by detecting the intensity of emitted bioluminescence light transients produced through the reaction of luciferin and activated platelet–released ATP. Geographically, we found that there were two distinct zones in the PRF scaffold in terms of bioluminescence intensity, known as the fibrin zone located at the upper half and the platelet-rich zone at the bottom half. Regarding PRF microstructure, some authors have revealed the inconsistency of scaffold compactness and porosities [
26,
30]. Therefore, we assume that the accumulation of activated platelets at the bottom half of PRF scaffold are attributed to the tighter compactness and smaller porosities of fibrin meshwork followed centrifugation. From functional perspective, the bottom-half platelet-rich zone was thus defined as the “PRF essence” region due to the relatively higher number of platelets. Despite the variations in PRF cytokine from batch to batch, the PRF essence consistently contains highly concentrated platelets and cytokines. When applied in vivo, we believe that the features of PRF essence may compensate for the individual discrepancy to facilitate tendinous healing.
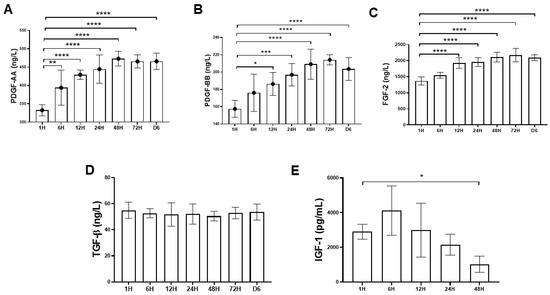
Since platelets aggregate along the fibrin fibers during clotting, the resultant three-dimensional (3D) scaffold could act as a reservoir of growth factors. Moreover, the equilateral junctions of the PRF 3D matrix allow the establishment of a fine and flexible fibrin network that enable cytokines enmeshment [
14]. Despite the increase in the clinical use of the platelet concentrates such as PRP for local tissue healing and regeneration, little is known about the biomolecule characteristic of these therapeutics in terms of cytokine-release kinetics. In this study, growth factors such as PDGF (AA and BB isoforms), FGF-2, TGF-β1, and IGF-1 were chosen for analysis because there are basic cytokines identified in platelets that play crucial role in cell proliferation, differentiation, and chemotaxis [
5]. In this study, the time-sequential cytokine-release kinetics was revealed by determining the cytokine concentrations of PRF-immersed supernatant at different time points. It was demonstrated that most growth factors could be detected at the first hour. Interestingly, an increasing trend of PDGF-AA and BB, and FGF-2 release was detected even after six days. The results of this study were consistent with previous reports, suggesting that the PRF tightly packed fibrin fibers can exhibit a locking effect on the cytokines [
30,
31]. The results of this study also lend support to our hypothesis that PRF could serve as a cytokine-delivery vehicle that would sustainably deliver bioactive molecules in promoting tendinous healing.
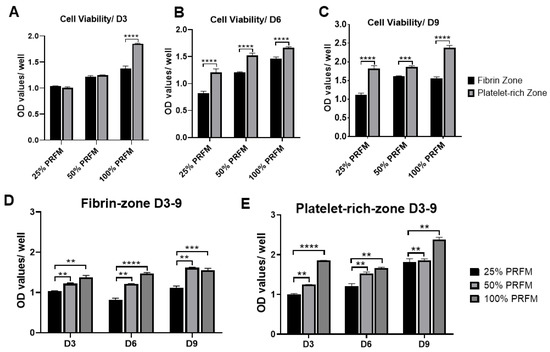
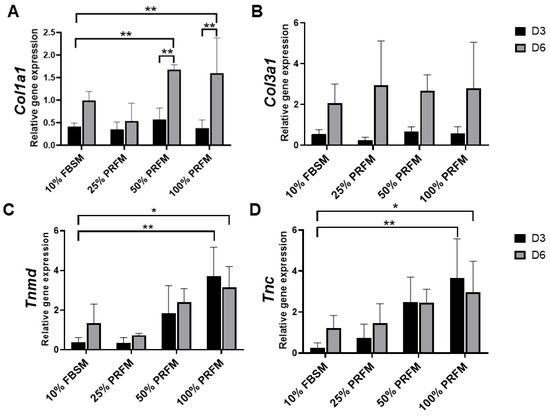
The functional healing of tendinous injury relied on the formation of tendinous tissue composed of sufficient cells with tenogenic potential. In this study, we found that PRF-conditioned medium (PRFM) could stimulate tenocytes proliferation and tenogenic matrix production. Based on the cytokine-release kinetic analysis, we found that the increased anabolic activities of tenocytes may be attributed to the cytokines originating from PRF. PDGF is a powerful mitogen for fibroblast which could increase cell density and proliferation [
32]. FGF could increase production of extracellular matrix while applied in vivo [
33]. When IGF-1 and TGF-β were delivered to the repair site of supraspinatus tendon-to-bone insertions of rats, increased cell proliferation, vascularity, and the production of fibrous repair tissue could be observed [
34]. Our results showed that the number of viable tenocytes treated with proportionally increased concentrations of PRFM (25%, 50%, and 100%) increased in a dose-dependent manner during the nine-day culture (). On the other hand, the expression of tendon-related genes such as
Tnmd and
Tnc were significantly upregulated after treatment with high-concentration PRFM.
Tnmd is a gene that is highly expressed in tendons and is required for tenocytes proliferation and tendon maturation [
35,
36,
37].
Tnc is a glycoprotein that plays an important role for tenocytes adaptation to compression [
38]. Moreover, PRFM has comparable effects as 10% FBSM on
Col1a1 and
Col3a1 gene expressions. Both types of collagen are important molecules in the tendon extracellular matrix and are produced in high quantity at the early phase of tendinous healing [
2,
3,
39]. Collectively, the in vitro results indicated that PRF exerts anabolic effects on tenocytes proliferation and tenogenic differentiation, which would benefit for in vivo tendinous healing.
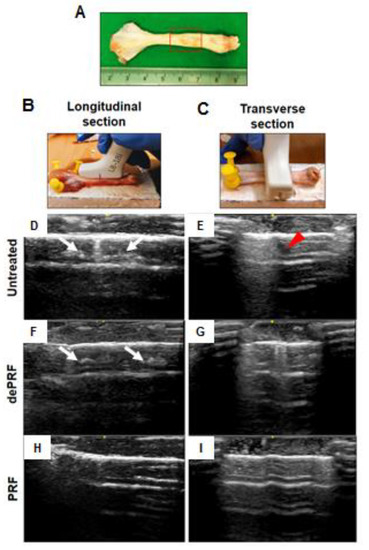
PRF is a biomaterial yielded by a natural polymerization process during centrifugation [
14]. Its natural fibrin architecture is beneficial for the containment and slow release of growth factors over time [
13,
30]. When implanted in vivo, the PRF scaffold could serve as a delivery platform for growth factors and provides an architecture for the attachment, proliferation, and migration of cells at the target site. The in vivo results demonstrated superior tendinous healing in the PRF-implanted group compared to control and the dePRF-implanted group, evidenced by sonographic and histological findings. For sonographic analysis, the intratendinous hyperechoic areas found in the control and dePRF groups may be ascribed to scar tissues comprised of different types of cells and an unorganized matrix. In contrast, the intratendinous morphology of the repaired tendon in PRF are mostly isoechoic. Moreover, the repaired tissue displayed a fibrillary appearance. These imaging findings indicate that the collagen fibers of the repaired tissue are cross linked and align along the long axis of the tendon, suggesting that the remodeling phase was underway.
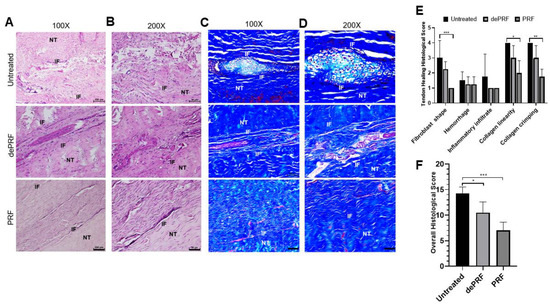
In general, tendon healing progresses through an inflammatory phase (days), followed by a reparative phase (weeks), and ended with a remodeling phase (months) [
4,
39]. Our histological results showed that PRF implantation leads to the initiation of a tendon reparative process characterized by increased tenocytes proliferation and differentiation. Despite the relatively high number of proliferative tenocytes found at the healing zone, most of the cells are spindly and elongated in shape. In addition, the collagen fibers were continuous, wavy, and aligned longitudinally in one direction. On the other hand, in control and dePRF rabbits, most of the tenocytes are oval/round in shape, resembling chondrocytes-like cells with basophilic lacanue. It was reported that the presence of cartilage is a reliable indicator of an inferior repair process and poorer biomechanics [
40]. Moreover, the fiber arrangements at the healing zone was loosed, disorganized, and fragmented without an identifiable pattern. Taken together, it was reasonably to propose that PRF could improve the reparative process by upregulating the tenocytes growth and collagen production. However, the histological findings also suggest that the remodeling process of tendinous injury is yet to be completed in terms of increased cell number and immature matrix synthesis.
Our data showed the potential of PRF in promoting Achilles tendon healing in a preclinical trial with an animal model. However, our study has several limitations. First, this preliminary study was conducted to evaluate the biological role of PRF in tendinous healing process at six weeks postoperatively. However, it might be more thorough to assess the surgical repair outcomes over different time points. The results also indicate that a longer study period may be needed to evaluate the complete course of tendinous healing.
- Beigi, R.; Kobatake, E.; Aizawa, M.; Dubyak, G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 1999, 276, C267–C278. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Jeon, S.H.; Park, J.Y.; Chung, J.H.; Choung, Y.H.; Choung, H.W.; Kim, E.S.; Choung, P.H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthr. Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef]
- Halpern, B.C.; Chaudhury, S.; Rodeo, S.A. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J. 2012, 8, 137–145. [Google Scholar] [CrossRef]
- Siess, W. Molecular mechanisms of platelet activation. Physiol. Rev. 1989, 69, 58–178. [Google Scholar] [CrossRef]
- Bai, M.Y.; Wang, C.W.; Wang, J.Y.; Lin, M.F.; Chan, W.P. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo) 2017, 72, 116–124. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Zaegel, M.; Das, R.; Harwood, F.L.; Silva, M.J.; Amiel, D.; Sakiyama-Elbert, S.; Gelberman, R.H. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J. Orthop. Res. 2007, 25, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014, 9, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.N.; Kim, H.M.; Sakiyama-Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Dex, S.; Alberton, P.; Willkomm, L.; Sollradl, T.; Bago, S.; Milz, S.; Shakibaei, M.; Ignatius, A.; Bloch, W.; Clausen-Schaumann, H.; et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. EBioMedicine 2017, 20, 240–254. [Google Scholar] [CrossRef]
- Dex, S.; Lin, D.; Shukunami, C.; Docheva, D. Tenogenic modulating insider factor: Systematic assessment on the functions of tenomodulin gene. Gene 2016, 587, 1–17. [Google Scholar] [CrossRef]
- Docheva, D.; Hunziker, E.B.; Fassler, R.; Brandau, O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Martin, J.A.; Mehr, D.; Pardubsky, P.D.; Buckwalter, J.A. The role of tenascin-C in adaptation of tendons to compressive loading. Biorheology 2003, 40, 321–329. [Google Scholar]
- Docheva, D.; Muller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug. Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Rosenbaum, A.J.; Wicker, J.F.; Dines, J.S.; Bonasser, L.; Razzano, P.; Dines, D.M.; Grande, D.A. Histologic stages of healing correlate with restoration of tensile strength in a model of experimental tendon repair. HSS J. 2010, 6, 164–170. [Google Scholar] [CrossRef]