The selenium (Se) is a crucial trace element for many living organisms, including soil microorganisms, plants, and animals including humans. The Se is taken up in the living cells of microorganisms, plants, animals, and humans in several inorganic forms such as selenate, selenite, elemental Se, and selenide. Biofortification of Se would help mitigation of Se deficiency in humans and animals.
- selenium
- trace element
- nutrition
- humans
- animals
- plants
- biofortification
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Selenium (Se) is a micronutrient essential for the proper functioning of plants and animals [1,2]. It was first described by the Swedish chemist Jacob Berzelius in 1817, however, its biological role was not determined until the 1950s when overdoses were linked to cardiac muscle dystrophy and acute hepatic necrosis [3]. In 1973, the beneficial biological role of Se as a key constituent of glutathione peroxidase (GPx) was discovered; thus it contributes to protecting the body from stress-induced oxidative damage to living cells. Subsequently, it was found that Se is not only associated with GPx, it is also connected with numerous other enzymatic activities within organisms’ cells; for example, Se biological forms like SeCys and SeMet are key constituents of iodothyronine deiodinase, which is crucial in the healthy functioning of the endocrine system [1,4].
It is estimated that globally one billion people are facing Se insufficiency [5] since Se is essential for the good functioning of the human immune, endocrine and reproductive systems, and also links with the function of the human brain [6]. An earlier study revealed that prolonged Se deficiency in the human body negatively affects the cardiovascular system and can lead to myocardial infarction, i.e., heart attacks [7]. Besides these examples, Se deficiency is also associated with Keshan and Kashin-Beck diseases, which predominantly occur in children and women of child-bearing age [8,9].
Generally, Se is uptaken by humans through consumption of Se-enriched plant and animal products, in particular from plant sources. Plants take up Se from the soil as selenates [10], which are then converted into the organic forms SeCys and SeMet [11]. Increasing the concentration of Se in edible plants’ parts/products is a viable pathway to overcome human Se deficiency. Recently, Se-biofortification combined with improved agronomic, breeding, molecular, biotechnology, and genetic engineering approaches have been recognized as a leading tool to enrich plant-food products with Se. While there is a large, disperse body of literature on the roles and mechanism of Se in human health and its enrichment in plant foods, a comprehensive review covering all these aspects is lacking.
2. The Natural Form of Selenium and Its Deficiency and Toxicity Symptoms
2.1. Natural Form
Selenium (Se) is widespread across the Earth and is present in the atmosphere, lithosphere, hydrosphere and biosphere [12]. The weathering of rocks and the eruption of volcanic gases are key sources of Se into the environment. Additionally, the decomposition of Se-enriched organic matter, through biomethylation by microorganisms, maintains a positive flow of this element into the atmosphere [1]. These mechanisms contribute to the presence of volatile Se compounds, viz., hydrogen selenide (H2Se), dimethyl selenide (DMSe), and selenium oxide (SeO2). Globally, the Se content in arable soils ranges between 0.33 and 2 mg/kg [13]. Se-rich areas are known as seleniferous areas [14]. The Se concentration in soils varies depending on the management of the local environment and the presence of both Se-rich parent materials and microorganisms necessary for its release into the atmosphere [15].
The soils which have originated from igneous rock, granite, sandstone, and limestone are all rich in Se [16]. Conversely, soils in temperate and humid climates are generally low in Se. Irrespective of soil depth, the Se content in mineral-enriched soils fluctuates ~14 mg/kg [17]. Se-rich materials include berzelianite (Cu2Se), klaustalite (PbSe), and naumanite (Ag2Se) [18]. Anthropogenic activities, such as the combustion of fossil fuels, metal smelting, international shipping and the over-use of inorganic fertilizers are primarily responsible for additional contributions of Se into the atmosphere (and thus into agricultural soils), from the before mentioned minerals [17].
Se-rich soils have been observed in the United States, Russia, parts of China, Australia, Canada, and Ireland [4]. In contrast, New Zealand and a wide portion of Europe have soils which are largely Se deficient [1]. Tomza-Marciniak [19] calculated that Se deficiencies exist in more than 70% of countries. However, the total Se content in soil is not a reliable estimate of plant available Se or the amount of Se available to humans and animals through plants [20]. The Se availability of plants is determined by a large number of soil chemical and biochemical characteristics, including sorption, disruption, soil pH, presence of other nutrients and methylation [21]. For example, Se uptake is higher for plants high in sulfur [22]. Se-rich foods include seafood, eggs, chicken, nuts, mushrooms, and green vegetables including spinach, cauliflower, and cabbage. Se concentrations in humans vary with agroclimatic region and daily diet.
In water, Se is present in minute quantities as selenates or selenites. Groundwater contains higher concentrations of Se than seawater [16,18]. This is largely the result of runoff of Se-rich fertilizer from intensively managed agricultural soils, as well as Se secretion from parent rock material [23]. In potable water, 10 µg Se per litre of water is acceptable according to the World Health Organization [24].
2.2. Selenium Deficiency Symptoms
2.2.1. Symptoms in Human
Prolonged Se deficiency adversely affects the cardiovascular system, which may be a cause of myocardial infarction [16]. Se deficiency also causes Keshan and Kashin-Beck diseases, which primarily occur in childbearing women and in children who live in areas deficient in Se [25]. A moderate deficiency of Se in daily food habit reduces immunity, can impair the nervous system and may cause congenital hypothyroidism in fetuses [26]. Additionally, Alzheimer’s disease, depression and anxiety have been associated with prolonged Se deficiencies [27]. Se may contribute to the suppression of HIV and slow the progression to AIDS [28]. Se is also necessary for the developing fetus in women and animals [16,29]. An insufficiency of Se in the human diet affects the thylakoid gland and may lead to moodiness and the impairment of behaviors and cognitive functions [30–32]. Se deficiency reduces the activity of 5′-thyronine deiodinase enzymes, leading to low triiodothyronine concentration in blood. Moreover, a deficiency of Se accelerates human ageing [31].
2.2.2. Symptoms in Animals
The majority of Se-deficiency symptoms have been observed in animals when less than 0.1 mg Se is present per kg of animal diet. Most commonly, Se deficiency causes a myo-degenerative syndrome [32] Table 1, also known as white muscle disease (WMD), whereby animal muscles are pale in appearance [33]. WMD may occur in all livestock including birds, and most seriously damages the skeletal (fibres/highly elongated cells) and cardiac muscles [34]. The deficiency of Se also impairs an animal’s immunity [35]. The clinical signs of Se deficiency in animals include reductions in appetite, fertility and growth, as well as muscle weakness [36]. Specific Se deficiency symptoms include heart disease in pigs, placenta retention in cows, and birds both a higher embryonic mortality rate and muscular dystrophy [33,37].
Table 1. Se responsive diseases in animals.
|
Syndrome |
Clinical Features |
|
White Muscle Disease |
Acute onset, stiffness, skeletal or cardiac muscles affected. |
|
Reproductive performance |
The retained fetal membrane in dairy cows. |
|
Abortion, Still-births |
Late third trimester abortions and stillbirths |
|
Myodegeneration of cattle (adult) |
Myocardial fibrosis, myoglobinuria weakness |
|
Infertility in cattle and sheep |
Decreased conception rate, early embryonic death |
|
Diarrhoea |
Diarrhoea, weight loss in young and adult cattle |
Source: Information in Table 1 was collected from Gupta and Gupta [32] with permission.
2.3. Selenium Toxicity
2.3.1. Toxicity in Humans and Animals
Both excessive and insufficiency of Se are detrimental to human health. The consumption of higher doses of Se can be toxic. There is a narrow limit between safe-and-adequate Se uptake and overconsumption leading to toxicity. There are only limited reports on human Se toxicity; this may be because many commonly consumed foods are Se non-accumulators. The symptoms of Se toxicity are hair loss and skin and nail lesions [38], hypotension, tachycardia, muscle contractions dizziness, nausea, vomiting, facial flushing, tremors and muscle soreness. In extreme cases, acute Se toxicity can cause serious intestinal and neurological problems, heart attack, kidney failure and death [39]. Excessive Se uptake can also damage the mucus membranes of the digestive tract, and lead to ongoing nausea, diarrhoea and also increase risk of type 2 diabetes [38]. In the case of animals, the death of poultry due to Se toxicity in wheat grains used as chicken feed was reported in South Dakota, USA [40].
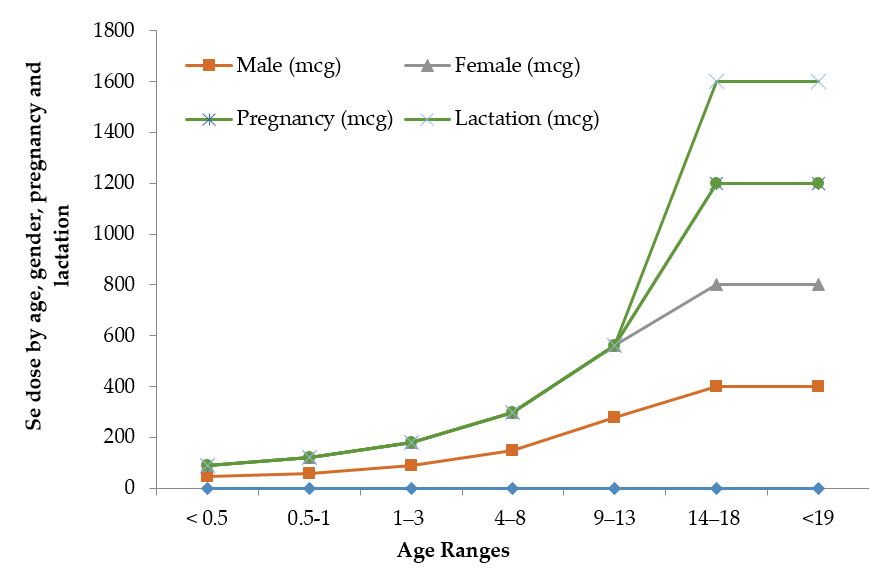
The limit between safe and toxic amounts of Se is small and has yet to be standardized in many geographical locations. The recommended dietary allowance of Se varies in humans with age, gender, pregnancy and lactation Figure 1. Pregnant or lactating women require higher 9% and 27% daily amounts of Se than other women [41]. Human beings must consume around 55 micrograms of Se per day and not exceed the maximum limit of 400 micrograms per day [42]. The World Health Organization recommends a daily average intake of 55 µg of Se, while the recommendation is varied with age, gender, diet and geographic location [43]. The International Food and Nutrition Board has recommended an average daily intake of 40–70 µg Se and 45–55 µg Se for men and women, respectively, and 25 µg Se for children [2,44]. A daily dose of 55–200 µg of Se is recommended for healthy adult humans [45,46]. Table 2 illustrates the maximum recommended daily Se intake limits, above which symptoms of Se-toxicity such as significant hair loss and abnormal nail growth appear [41].
Figure 1. Recommended maximum daily dietary allowances for Se.
Table 2. Recommended maximum daily Se intake levels.
|
Age |
Male |
Female |
Pregnancy |
Lactation |
|
Birth to 6 months |
15 mcg * |
15 mcg * |
|
|
|
7–12 months |
20 mcg * |
20 mcg * |
|
|
|
1–3 years |
20 mcg |
20 mcg |
|
|
|
4–8 years |
30 mcg |
30 mcg |
|
|
|
9–13 years |
40 mcg |
40 mcg |
|
|
|
14–18 years |
55 mcg |
55 mcg |
60 mcg |
70 mcg |
|
19–50 years |
55 mcg |
55 mcg |
60 mcg |
70 mcg |
|
51+ years |
55 mcg |
55 mcg |
|
|
Source: The information in Table 2 is collected from IMFNB [41] with permission. * Breast milk, formula, and food should be the only sources of selenium for infants.
In case of the available forms Se, Ríos et al. [47] regarded that >40 μmol Se/L of selenate as toxic, while Hawrylak-Nowak [48] estimates that safe concentrations of Se are between 20 and 15 μmol/L for selenate and selenite, respectively. However, Ríos et al. [49] reconsidered and set an upper limit of 80 μmol Se/L.
2.3.2. Selenium Phytotoxicity
Toxic levels of Se within plant tissues are greater than 5 mg/kg [50]. Se toxicity does not occur when selenate is applied at rates between 10–200 g Se per ha, which are commonly recommended for wheat biofortification. Se is usually not considered essential for taller plants, while soils low in Se are considered to neither inhibit plant growth nor reduce crop yield [50,51]. However, some research has indicated the beneficial effects of low doses of applied Se on crop performance. For example, increased growth of ryegrass (Lolium perenne) and lettuce (Latuca sativa) was recorded when crops were fertilized with Se and exposed to UVB radiation. Low-dose applications of Se are useful for crop plants only when they are under oxidative stress, otherwise, this microelement is not essential.
References
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298, doi:10.3390/molecules24071298.
- Galan-Chilet, I.; Tellez-Plaza, M.; Guallar, E.; De Marco, G.; Lopez-Izquierdo, R.; Gonzalez-Manzano, I.; Carmen Tormos, M.; Martin-Nuñez, G.M.; Rojo-Martinez, G.; Saez, G.T.; et al. Plasma selenium levels and oxidative stress biomarkers: A gene–environment interaction population-based study. Free Radic. Biol. Med. 2014, 74, 229–236.
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775.
- Kieliszek, M.; Błazejak, S. Current knowledge on the importance of selenium in food for living organisms: A Molecules 2016, 21, 609, doi:10.3390/molecules21050609.
- Nothstein, A.K.; Eiche, E.; Riemann, M.; Nick, P.; Winkel, L.H.E.; Göttlicher, J.; Steininger, R.; Brendel, R.; Brasch, M.V.; Konrad, G.; et al. Tracking se assimilation and speciation through the rice plant–nutrient competition, toxicity and PLoS ONE 2016, 26, e0152081.
- Pilon-Smits, E.A.; Le Duc, D.L. Phytoremediation of selenium using transgenic plants. Opin. Biotechnol. 2009, 20, 207–212, doi:10.1016/j.copbio.2009.02.001.
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Environ. Saf. 2018, 149, 291–306.
- McCann, J.C.; Ames, B.N. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: Why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011, 25, 1793–1814.
- Shreenath, A.P.; Ameer, M.A.; Dooley, J. Selenium Deficiency. In Treasure Island (FL): StatPearls Publishing; 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482260/ (accessed on 30 January 2021).
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Selenium uptake in Zea mays supplied with selenate or selenite under hydroponic conditions. Plant Soil 2013, 362, 107–117.
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299.
- Reich, H.J.; Hondal, R.J. Why nature chose selenium? ACS Chem. Biol. 2016, 11, 821–841.
- Mason, R.P.; Soerensen, A.L.; DiMento, B.P.; Balcom, P.H. The global marine selenium cycle: Insights from measurements and modeling. Biogeochem. Cycles 2018, 32, 1720–1737.
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 375–
- Winkel, L.H.; Trang, P.T.K.; Lan, V.M.; Stengel, C.; Amini, M.; Ha, N.T.; Viet, P.H.; Berg, M. Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. Natl. Acad. Sci. USA 2011, 108, 1246–1251.
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Pollut. 2018, 234, 915–934.
- Söderlund, M.; Virkanen, J.; Holgersson, S.; Lehto, J. Sorption and speciation of selenium in boreal forest soil. Environ. Radioact. 2016, 164, 220–231.
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Res. 2018, 164, 288–301.
- Tomza-Marciniak, A.; Bąkowska, M.; Pilarczyk, B.; Semeniuk, M.; Hendzel, D.; Udała, J.; Balicka-Ramisz, A.; Tylkowska, A. Concentration of selenium in the soil and in selected organs of roe deer (Capreolus capreolus) from the Greater Poland Voivodeship. Acta Sci. Pol. Zootech. 2010, 9, 251–260.
- Chilimba, A.D.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop. Res. 2012, 125, 118–128.
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium behavior in the soil environment and its implication for human health. Ciência e Agrotecnologia, 2017, 41(6), 605-615.
- Stroud, J.; Broadley, M.; Foot, I.; Fairweather-Tait, S.; Hart, D.; Hurst, R.; Knott, P.; Mowat, H.; Norman, K.; Scott, P. Soil factors affecting selenium concentration in wheat grain and the fate and speciation of Se fertilisers applied to soil. Plant Soil 2010, 332, 19–30.
- Paikaray, S. Origin, mobilization and distribution of selenium in a soil/water/air system: A global perspective with special reference to the Indian scenario. Clean 2016, 44, 474–487.
- Selenium in Drinking-Water. In Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; World Health Organization (WHO/SDE/WSH/03.04/13): Geneva, Switzerland, 2003.
- Oropeza-Moe, M.; Wisløff, H.; Bernhoft, A. Selenium deficiency associated porcine and human cardiomyopathies. Trace Elem. Med. Biol. 2015, 31, 148–156.
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Biochem. Biophys. 2013, 536, 152–157.
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239.
- Lipinski, B. Rationale for the treatment of cancer with sodium selenite. Hypotheses 2005, 64, 806–810.
- Okunade, K.S.; Olowoselu, O.F.; Osanyin, G.E.; John-Olabode, S.; Akanmu, S.A.; Anorlu, R.I. Selenium deficiency and pregnancy outcome in pregnant women with HIV in Lagos, Nigeria. J. Gynecol. Obstet. 2018, 142, 207–213.
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and thyroid disease: From pathophysiology to treatment. J. Endocrinol. 2017, 1297658.
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep. 2019, 27, 2785–2797, doi:10.1016/j.celrep.2019.05.001.
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Plant Sci. 2017, 7, 2074, doi:10.3389/fpls.2016.02074.
- Underwood, E.J.; Suttle, N.F. The mineral nutrition of livestock. In Selenium; Underwood, E.J., Suttle, N.F., Eds.; CABI Publishing: New York, NY, USA, 1999; pp. 421–
- Whanger, P.D.; Weswig, P.H.; Oldfield, J.E.; Cheeke, P.R.; Muth, O.H. Factors influencing selenium and white muscle disease: Forage types, salts, amino acids and dimethyl sulfoxide. Rep. Int. 1972, 6, 21–37.
- Turner, R.J.; Finch, J.M. Selenium and the immune response. Nutr. Soc. 1991, 50, 275–285.
- Combs, Jr. G.F.; Combs, S.B. The Role of Selenium in Nutrition; Academic Press: Cambridge, MA, USA, 1986; p. 532.
- Andrews, E.D.; Hartley, W.J.; Grant, A.B. Selenium- responsive diseases of animals in New Zealand. New Zealand Vet. 1968, 16, 3–17.
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Total. Environ. 2008, 400, 115–141.
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Lett. 2014, 230, 295–303.
- Franke, K.W. A new toxicant occurring naturally in certain samples of plant foodstuffs. Results obtained in preliminary feeding trials. J. Nutr. 1934, 8, 597–609.
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Cambridge, MA, USA, 2000; pp. 1–20.
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere 2017, 188, 650–658.
- Post, M.; Lubiński,W.; Lubiński, J.; Krzystolik, K.; Baszuk, P.; Muszyńska, M.; Marciniak, W. Serum selenium levels are associated with age-related cataract. Agric. Environ. Med. 2018, 25, 443–448.
- Pophaly, S.D.; Singh, P.; Kumar, H.; Tomar, S.K.; Singh, R. Selenium enrichment of lactic acid bacteria and bifidobacteria: A functional food perspective. Trends Food Sci. Tech. 2014, 39, 135–145.
- USDA-ARS. USDA National Nutrient Database for Standard Reference, Release 25 USDA-ARS Washington, DC. 2002. Available online: http://www.nal.usda.gov/fnic/selenium (accessed on 30 January 2021).
- Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks, 2009. Available online: http://www.who.int/healthinfo/global_burden_disease/ GlobalHealthRisks _report annex.pdf (accessed on 28 January 2021).
- Ríos, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce Sci. Hortic. 2008, 116, 248–255.
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Growth Regul. 2013, 70, 149–157, doi:10.1007/s10725-013-9788-5.
- Ríos, J.J.; Blasco, B.; Leyva, R.; Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Romero, ; Ruiz, J.M. Nutritional balance changes in lettuce plant grown under different doses and forms of selenium. J. Plant. Nutr. 2013, 36, 1344–1354.
- Reilly, C. Selenium in Food and Health; Blackie: London, UK, 1996.
- Shrift, A. Aspects of selenium metabolism in higher plants. Ann Rev Plant Physiol. 1969, 20, 475–494.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26040881