4D printing is the fabrication process of 3D objects that can change their shape over time or in response to an environmental stimulus. This process demonstrates a radical shift in additive manufacturing.
- 4D printing
- additive manufacturing
1. Introduction
Additive manufacturing (AM), commonly known as three-dimensional (3D) printing, is a popular fabrication technique due to its ability to create complex, customizable structures from a 3D computer-aided design (CAD) file [1]. It is an attractive alternative to traditional fabrication processes (e.g., moulding and machining) due to the reduction in both difficulty and cost of producing detailed customizable architectures [2]. Developments since the introduction of 3D printing in 1984 have been improved fabrication accuracy, speed, multiple materials, and costs [3]. Nevertheless, an inherent shortfall of these structures is their static and rigid nature; retaining the shape in which they were originally printed and generally only performing one function [4]. The drive to incorporate active materials into the 3D printing process to overcome these limitations has led to the development of four-dimensional (4D) printing technologies to create dynamic structures [1].[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45]
4D printing is the fabrication process of 3D objects that can change their shape over time or in response to an environmental stimulus. This process demonstrates a radical shift in additive manufacturing [5,6]. It offers a streamlined path from idea to reality with performance-driven functionality built directly into the materials [5]. With this technique, a wide range of active programmable materials can be produced which have the capability to self-transform from one shape to another [5].
Systems that respond autonomously to a change in their environment are commonly found in nature, for example, the nastic movement of leaves and flowers can be triggered by humidity, light, or touch [7]. This property had not, however, yet been achieved in manufactured objects until recently [8]. At the core of this research is the development of additive manufacturing. Printing methods using smart materials to produce four-dimensional architectures and metamaterials. These three-dimensional structures are dynamic and have the ability to self-transform in response to a predetermined environmental stimulus, such as electricity, light, temperature, or moisture, hence creating a fourth dimension of time [9]. The shape-changing characteristics of these structures derive from the use of stimuli-responsive smart materials during the printing process, which give the structure the ability to change its function, shape, or physical properties such as Young’s modulus to form selective structures and configurations [1,10,11,12]. This review focuses on dynamic structures with shape-changing abilities. The characteristic differences between 3D and 4D printing are given in Table 1 and Figure 1.
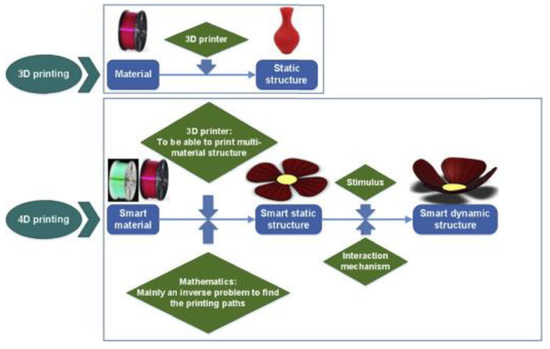
Figure 1. The key differences between 3D and 4D printing. 3D printing involves the deposition of material into a predetermined static shape. 4D printing, on the other hand, involves the careful deposition of a smart material into a predetermined, smart static structure. When this smart static structure interacts with an internal or external stimulus, it will transform its shape and become a smart, dynamic structure [13].
Table 1. Characteristic differences between 3D and 4D printing technologies. Adapted from [12].
| Characteristics | 3D Printing | 4D Printing |
|---|---|---|
| Build Process |
|
|
| Materials |
|
|
| Shape flexibility |
|
|
| Shape-memory programming |
|
|
| Applications |
|
|
First introduced in 2013, 4D printing has since received great interest within material science showing potential for application within the fields of soft robotics, defence, and manufacturing, among others [14]. Fabricating 4D structures for use in tissue engineering and drug delivery systems provides a promising prospective technology for future generations, and hence this review will focus on biomedical applications [15]. This technology has the potential to supplement, or even replace, devices used in various surgical procedures, including skin grafts or organ donations. A desirable characteristic of smart materials is their ability to deform into a temporary configuration and recover to their original form by varying the applied stimulus [11]. This is called the two-way shape memory effect (SME) and has been exploited by material scientists to produce objects that can be actuated after printing [16,17]. Research developments have been successful in developing the SME to produce hierarchical self-morphing structures that can adopt multiple spatial configurations in response to a varying stimulus [18]. The structural response is dependent on both the materials and techniques used in the printing process. The shape-morphing capability is usually achieved by either (1) printing a combination of active and rigid materials in different regions of the structure to create areas of differential strain; or (2) by programming the temporary shape into the thermo-mechanics of the structure after printing. An active area for research into the SME is incorporating the thermo-mechanic programming within the 3D printing process [8,19,20]. The most suitable method will vary depending on the printing materials used and the desired structural response. Current smart materials deemed suitable include shape memory polymers (SMPs), hydrogel composites, shape memory alloys (SMAs), and shape memory composites (SMCs). However, while shape-memory materials seem to have been widely researched within material science, their conjunction with 3D printing is a relatively recent venture. Most AM methods such as Stereolithography (SLA) and Fused Deposition Modelling (FDM) involve the sequential deposition of layers of material onto a building platform [21]. These processes can fabricate devices on the nano/micro scale showing potential for use in drug delivery systems (DDS) and minimally invasive surgical systems [22]. The potential for this technology to develop customizable dressings, drug delivery systems (DDS). Through the addition of the fourth dimension, 4D printing is seen as being particularly well-suited to the biomedical field, with current research focusing on drug delivery systems (DDSs), tissue engineering, regenerative medicines, and biomimicry [23].
2. Additive Manufacturing Techniques
The 3D printing technology (also referred to as AM) is used to generate a 3D specimen in which layers of material are continuously formed under a computer-controlled program to create a physical object. ISO/ASTM52900-15 defines seven categories of Additive Manufacturing (AM) processes: material extrusion, vat photopolymerization, powder bed fusion, material jetting, binder jetting, sheet lamination, and directed energy deposition [24]. The main commercially available 4D additive manufacturing processes have been broadly categorised by their associated printing mechanisms; liquid solidification, powder solidification, and direct material extrusion [25]. These methods involve the light-curing of a photopolymer, melt-material extrusion, and direct-ink printing [26]. The technique is chosen depending on both the smart materials to be printed and the desired properties/function of the final structure. Parameters such as printing speed, laser frequency, and nozzle temperature directly affect fabrication accuracy, and hence these must be investigated and optimised to ensure the viability of scale-up for industrial manufacture. The printing process can also be chosen to enhance and facilitate the shape-memory functionality of the object. Independent of the AM technique, to fabricate a 3D structure requires a detailed Computer Aided Design (CAD) model of the physical architecture. In most cases, the design model is digitally sliced into thin horizontal layers, and the printer forms the structure by sequentially printing each layer of the material [27]. The basic principles of commercial AM technologies are shown in Figure 2.
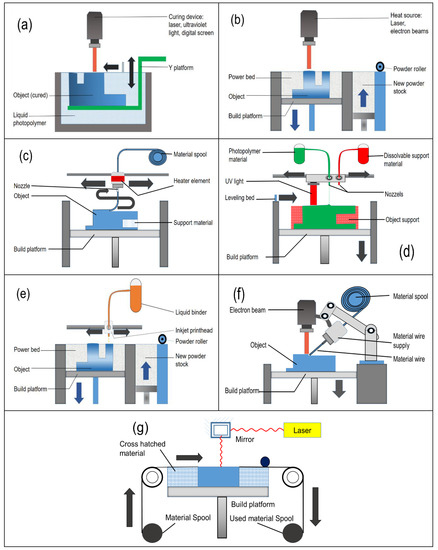
Figure 2. Different Additive manufacturing (AM) technologies used in 3D printing. (a) Photopolymerization; (b) Power bed fusion; (c) Material extrusion; (d) Material jetting; (e) Binder jetting; (f) Direct energy deposition; and (g) Sheet lamination. AM technologies currently used in 4D printing are fused deposition modelling (FDM); selective laser sintering (SLS); stereolithographic apparatus (SLA); and polyjet.
2.1. Vat Polymerization
This area of AM technology requires the use of a liquid photopolymerizable resin, which is hardened by curing with light layer-by-layer to fabricate the solid 3D structure. The main light-based techniques used in 4D printing are vat photopolymerization and photojetting.
2.1.1. Stereolithography (SLA)
In this technique, a monomer resin held in a vat is exposed to a UV light source, causing a localised polymerisation reaction that hardens the resin. When a layer is cured, the build-platform moves the structure to expose a fresh layer of resin to the UV light [26]. The light source can either be directed from above, known as “bottom-up” (Figure 3a), or from below through a transparent window called “top-down” (Figure 3b) [28]. Repeating these steps until the final layers are cured produces the solid 3D structure [29,30].
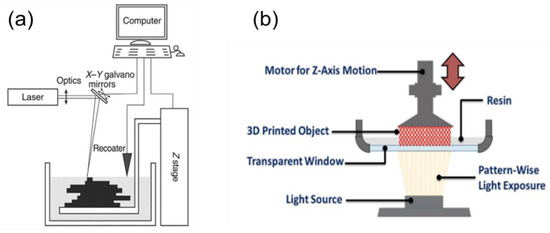
Figure 3. Photopolymerization fabrication techniques. (a) The light source is from above and is taken from [30]. (b) The light source is coming from below and is taken from [26].
Invented by Charles W. Hull in the late 1980s as the first commercially available 3D printing technology, stereolithographic apparatus (SLA) was initially adopted as an inexpensive and efficient way to manufacture prototypes and customisable designs [5,31]. SLA is now amongst the most widely used solid freeform fabrication techniques [28,32,33]. This AM process requires the use of liquid photopolymerisable and cross-linkable resins [34] and benefits from using materials that can achieve high curing rates and precise depositions when printed [32]. These material restrictions and the time-intensive nature of the vertical build-up of layers remain the major limitations of SLA as a 3D printing technology.
A major advantage of SLA is the ability to fabricate high-resolution objects of various sizes; submicron-scale to decimetre-sized objects have been produced using this method [28]. While most AM techniques can achieve structural details in the magnitude of 50–200 micron, Melchels et al. report the ability of SLA to produce details as small as 20 μm [28], and Boydston et al. have SLA-printed SMPs with accuracy between 0.1 mm and 1 μm [26]. This indicates the suitability of stereolithography for the fabrication of intricate biomedical devices where small, detailed structures are required for deployment within the body.
The area of liquid photopolymerizable smart materials is in its infancy, with only a small fraction of those available being biocompatible and therefore suitable for biomedical use [28,34]. Research efforts are being made both to enhance the properties of those already available and to discover new ones. For example, a review by Melchels et al. reports various biomaterials suitable for use with SLA to create porous structures for tissue engineering applications [28]. SLA is also suitable for multi-material applications and has been utilised by Arcaute et al. to fabricate shape-memory composites (SMCs) [34]. SLA and other light photopolymerization-based techniques provide an accurate and simple fabrication process for creating dynamic architectures. If further developments can be made to improve printing speeds, this technique shows potential as a method for mass-manufacture of intricate 4D structures for biomedical applications.
2.1.2. Digital Light Processing (DLP)
Another light-based AM technique with the potential to fabricate biomedical devices is a digital light projection (DLP). This technology utilises a digital mirror device (DMD) containing several million mirrors. A 2D pattern of pixels is projected onto the mirror, which allows instantaneous polymerisation of the entire resin, as shown in Figure 4. By rotating the digital mirror device (DMD) and breaking contact with the light source the device can be turned on/off. The print times are only dependent on layer thickness and exposure times since the entire layer is cured at once [28].
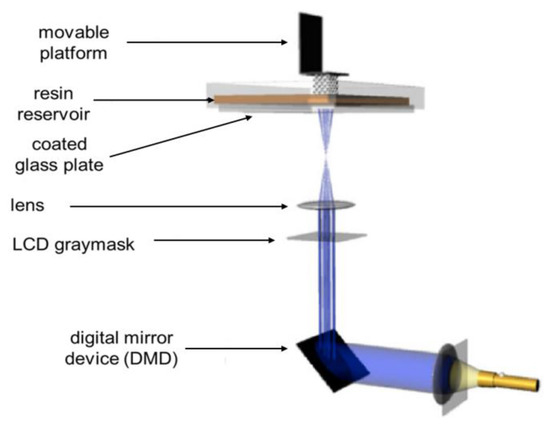
Figure 4. Top-down digital light processing AM technique. Taken from [27].
DLP is a suitable technique for fabricating SMPs, as recently evidenced by Invernizzi et al., who 4D printed a new thermo-responsive SMP material comprising of polycaprolactone (PCL) chains with cross-linked 2-ureido-4 [1H]-pyrimidinone (UPy) monomer units. DLP was chosen as an inexpensive fabrication technique, and the researchers were able to create a structure with self-healing capabilities suitable for biomedical applications [35].
2.2. Powder Bed Fusion
The basic principle of powder bed fusion AM techniques is the use of heat to melt or fuse a material together [26]. The main techniques in this area are selective laser sintering (SLS) and selective laser melting (SLM), which melt powders of polymers and metals, respectively [36]. These techniques do not require the use of any supports due to the unsintered powder compacted around the structure [37].
2.2.1. Selective Laser Sintering (SLS)
Selective Laser Sintering (SLS) is a similar technique to SLA, however, a high-powered laser is used to sinter a photopolymer powder rather than a liquid resin [37]. The newly formed layer is formed by sintering of the powder by an incident laser beam. A levelling roller is used to spread a fresh layer of powder over the previously formed layer, and the unsintered powder acts as a support for the overhanging layers [30]. The process of powder rolling and sintering is repeated until the final 3D structure is formed. A disadvantage of this technique is that the formed structure requires thorough cleaning to remove excess powder and the high temperatures involved mean this technique is not currently suitable for bioprinting [38]. Current 3D applications for this technology include the printing of hearing aid shells. Its ability to print biomaterials indicates its potential to fabricate various personalised medical devices [34,39].
2.2.2. Selective Laser Melting (SLM)
Selective Laser Melting (SLM) uses a laser to melt metallic powders in the same layer-by-layer process by inter-stage curing from a high-intensity laser beam [40]. This creates a homogeneous and dense 3D metallic structure removing the need for structural supports or binders [26]. The printing set-up is enclosed in a chamber as the reactivity of metallic compounds requires an inert atmosphere [40]. The 4D potential of this technology derives from the ability to fabricate both shape-memory alloys (SMAs) and single metallic smart materials [1]. For example, Shishkovsky et al. recently fabricated structures made from the shape memory alloys Ni-Ti (Nitinol) and Cu-Ni-Al using SLM [40]. Figure 5 displays the general apparatus for SLM and SLS AM techniques.
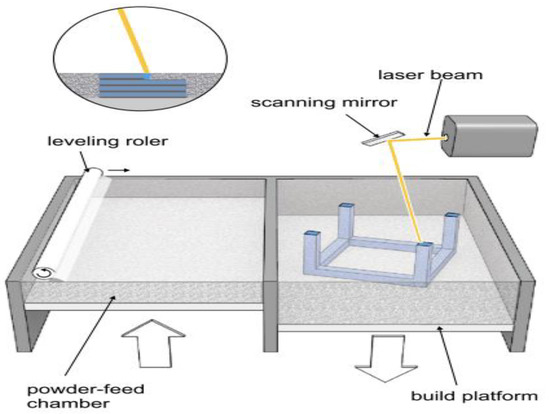
Figure 5. Apparatus for heat-based AM techniques selective laser melting (SLM)/selective laser sintering (SLS). Taken from [25].
2.3. Material Extrusion
2.3.1. Direct Ink Writing (DIW)
Direct ink writing involves controlling the orientation of an anisotropic filler within a polymer matrix. This generates stresses which are manipulated sequentially for individual pixels using ink writing. Although the time-intensive material layering and curing of light-based techniques is omitted, the pixel-after-pixel manipulation also results in slow fabrication times [2].
Slow printing times remain a major limitation, and hence an area of extensive research within both 3D and 4D printing technology. The layered process of the fabrication methods is slow and hinders the potential for wide-scale manufacture. A solution to this has been proposed by Huang et al., who reported a potential ultrafast 4D printing technique where light-curable monomers are briefly exposed to digital light, removing the need for sequential layering or manipulation of pixels. Short bursts of light exposure caused the pixels within a 2D monomer film to polymerise to different extents resulting in varying crosslinking densities throughout the material. This produced controllable differential swelling and stresses within the printed structure, which induced 3D shape morphing capabilities of the SMP and hydrogel when immersed in water. The cross-linking densities of the smart material can be tailored by controlling the digital light exposure. Huang et al. report that this simple technique has the potential to fabricate complex geometries with shorter fabrication times because of the controllable stresses and short light exposure [2].
2.3.2. Fused-Deposition Modelling (FDM)
Fused-deposition modelling (FDM), also known as melt material extrusion (MME) or fused filament fabrication (FFF), is an AM technique based on the extrusion of thermoplastic filaments [26]. A reel of polymer filament is melted to form a semi-liquid before being extruded through a heated nozzle. The partially melted filaments solidify when deposited onto the build platform, and the 3D structure is built up from sequential layering of the extruded filaments [39]. A schematic for the mechanism (Figure 6a) and apparatus (Figure 6b) of this technique is shown below.
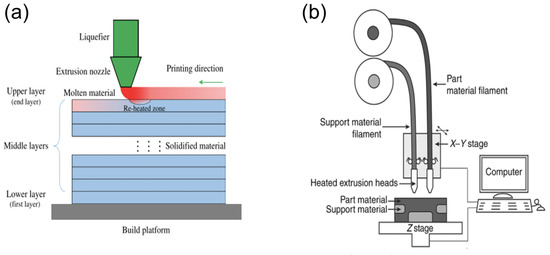
Figure 6. Fused-deposition modelling (FDM) extrusion-based AM technique. (a) Cross-section of printed material taken from [41]; (b) schematic of general apparatus taken from [30].
Filaments suitable for use in FDM have been produced from various thermoplastics, including polylactic acid (PLA), acrylonitrile butadiene styrene copolymer (ABS), polycarbonate (PC), and polyurethane (PU), which each exhibit variable stiffness, elasticity, and toughness [26]. FDM printers are simple, inexpensive, and reliable [42] with the potential to fabricate various medical devices such as modified release dosage forms for drug delivery systems, as evidenced by Goyanes et al. [39]. Bodaghi et al. have also utilised FDM to create a structure with triple-SME using a combination of hot and cold programming of an SMP [43].
Due to the high printing temperatures required, FDM can only be used with heat-resistant materials. It is therefore not suitable for printing cell-laden bioinks or hydrogels, which become denatured when exposed to high temperatures [42]. FDM is an interesting fabrication technique in the field of tissue engineering due to the potential for creating porous polymer scaffolds [27]. FDM is also unsuitable for fabricating polymers with low glass transition temperatures (Tg). Polymer filaments with low Tg lose their stiffness at ambient temperatures making extrusion through the printing nozzle almost impossible [42]. This can be prevented either by employing materials with higher glass transition temperatures or operating at temperatures far below Tg [42]. For example, a study by Kashyap et al. investigated the process of combining FDM with salt leaching to create a radiopaque, porous SMP structure with potential for use within interventional radiology [42]. The addition of fillers (Tungsten as a radiopaque agent and sodium chloride as a porogen for salt leaching) in the printing filament reduced the printability of the polymer due to increased viscosity, causing blockage of the printing nozzle. The researchers suggested incorporating a larger diameter printing nozzle to reduce blockage, but this reduced the precision and accuracy of the printed structure [42]. The group considered that using filaments of higher stiffness at ambient conditions, hence polymers with higher Tg, could increase the pushing force and reduce blockage [42]. Extensive research is being focused on finding suitable materials for fabricating biomedical devices with incorporated shape-memory behaviour. Developments in the last decade have vastly reduced the cost of FDM printers. This supports the prospect of FDM as an inexpensive option for producing personalised medical devices such as drug delivery systems.
2.4. Material Jetting
In recent years there have been vast developments in 4D printing technologies, most notably the Photopolymer Inkjet (PolyJet) printer, which employs the photo jetting principle. Photo jetting is a 4D printing process whereby microscopic layers of resin are jetted onto the build platform. The resin is instantly cured by UV light before the next layer is deposited on top [10,26]. Recent developments have expanded PolyJet technology to facilitate multi-material printing. This works by concurrent extrusion of distinct materials through different nozzles in the apparatus. Printing both active and inactive materials in distinct areas of a structure can create hinges and joints, resulting in origami-inspired shapes that can self-fold, twist, and curl when exposed to the environmental stimulus as reported by Ge et al. [44].
Light-based printing methods such as SLA, DLP, and Polyjet, where a photopolymerizable ink is cured by light, are attractive due to their ability to fabricate detailed structural designs. There have been notable efforts into finding biocompatible liquid photopolymerizable materials, however, further research is required before there can be a wide-scale application of this technology in fabricating biomedical devices.
2.5. Microscopy Aided Design and Manufacture (MADAME)
Sidler et al. recently published a report detailing a new printing technology with potential use in fabricating wearable technologies and internal biomedical devices [15]. This technique uses multi-dimensional printing incorporated with programmable weaving to fabricate complex structures such as woven protein fibres. An interesting application of this technology is the fabrication of smart textiles for wound treatments. The textiles are tuned to individual patients’ movements, can administer drugs, and can signal to the patient or carer when replacement of the textile is required. This method has further potential to produce smart dressings, drug delivery patches, and replacement body parts [15]. This study highlights the current drive to improve AM printing techniques for the biomedical industry. Table 2 displays a summary of the main AM techniques and smart materials currently used to fabricate 4D structures.
Table 2. Summary of common 4D AM techniques and applicable smart materials. Adapted from [45].
| AM Process | AM Systems | Applicable Materials | Ref. |
|---|---|---|---|
| Liquid solidification | SLA | SMPs | [32] |
| Soybean oil | [46] | ||
| SMCs | [34] | ||
| Direct laser printing (DLP) | SMPs | [35] | |
| Material Extrusion | FDM | SMPs | [26] |
| SMCs | [42] | ||
| Hydrogel extrusion | SMCs | [47] | |
| Material Jetting | PolyJet | SMPs | [48] |
| SMCs | [44,49] | ||
| Powder solidification | SLM | SMAs | [40] |
| SLS | SMPs | [38] | |
| SMCs | [44] |
This entry is adapted from the peer-reviewed paper 10.3390/su122410628
References
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122, doi:10.1080/17452759.2015.1097054.
- Huang, L.; Jiang, R.; Wu, J.; Song, J.; Bai, H.; Li, B.; Zhao, Q.; Xie, T. Ultrafast Digital Printing toward 4D Shape Changing Materials. Adv. Mater. 2017, 29, doi:10.1002/adma.201605390.
- Raviv, R.; Zhao, W.; Mcknelly, C.L.; Papadopoulou, A.; Kadambi, A.; Shi, B.; Hirsch, S.; Dikovsky, D.; Zyracki, M.; Olguin, C.; et al. Active printed materials for complex self-evolving deformations. Sci. Rep. 2014, 4, doi:10.1038/srep07422.
- Leist, S.K.; Gao, D.; Chiou, R.; Zhou, J. Investigating the shape memory properties of 4D printed polylactic acid (PLA) and the concept of 4D printing onto nylon fabrics for the creation of smart textiles. Virtual Phys. Prototyp. 2017, 12, 290–300, doi:10.1080/17452759.2017.1341815.
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Archit. Des. 2014, 84, 116–121, doi:10.1002/ad.1710.
- André, J.-C. From Additive Manufacturing to 3D/4D Printing 3: Breakthrough Innovations: Programmable Material, 4D Printing and Bio-printing, 1st ed.; John Wiley & Sons Incorporated: Hoboken, NJ, USA, 2018.
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418, doi:10.1038/nmat4544.
- Zarek, M.; Mansour, N.; Shapira, S.; Cohn, D. 4D Printing of Shape Memory-Based Personalized Endoluminal Medical Devices. Macromol. Rapid Commun. 2017, 38, doi:10.1002/marc.201600628.
- Ramesh, S.; Reddy, S.K.; Usha, C.; Naulakha, N.K.; Adithyakumar, C.; Reddy, M.L.K. Advancements in the Research of 4D Printing-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012123, doi:10.1088/1757-899X/376/1/012123.
- Bodaghi, M.; Damanpack, A.R.; Liao, W.-H. Self-expanding/shrinking structures by 4D printing. Smart Mater. Struct. 2016, 25, 105034.
- Shin, D.-G.; Kim, T.-H.; Kim, D.-E. Review of 4D printing materials and their properties. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 349–357, doi:10.1007/s40684-017-0040-z.
- Javaid, M.; Haleem, A. 4D printing applications in medical field: A brief review. Clin. Epidemiol. Glob. Health Sep. 2018, doi:10.1016/J.CEGH.2018.09.007.
- Al’Aref, S.J.; Mosadegh, B.; Dunham, S.; Min, J.K. 3D printing applications in cardiovascular medicine. 3D Print. Appl. Cardiovasc. Med. 2018, 1–278, doi:10.1016/C2015-0-00622-0.
- Miao, S.; Cui, H.; Nowicki, M.; Xia, L.; Zhou, X.; Lee, S.-J.; Zhu, W.; Sarkar, K.; Zhang, Z.; Zhang, L.G. Stereolithographic 4D Bioprinting of Multiresponsive Architectures for Neural Engineering. Adv. Biosyst. 2018, 2, doi:10.1002/adbi.201800101.
- Sidler, H.J.; Duvenage, J.; Anderson, E.J.; Ng, J.; Hageman, D.J.; Tate, M.L.K. Prospective design, rapid prototyping, and testing of smart dressings, drug delivery patches, and replacement body parts using Microscopy Aided Design and ManufacturE (MADAME). Front. Med. 2018, 5, 348, doi:10.3389/fmed.2018.00348.
- Shin,D.-G.; Kim, T.-H.; Kim, D.-E. Review of 4D Printing Materials and Their Properties. Int. J. Precis. Eng. Manuf. Technol. 2017, 4 (3), 349–357. https://doi.org/10.1007/s40684-017-0040-z.
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J.-M. Shape-memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294, doi:10.1016/j.eurpolymj.2016.05.004.
- Pei, E.; Loh, G.H. Technological considerations for 4D printing: An overview. Prog. Addit. Manuf. 2018, 3, 95–107, doi:10.1007/s40964-018-0047-1.
- Teoh, J.E.M.; An, J.; Chua, C.K.; Lv, M.; Krishnasamy, V.; Liu, Y. Hierarchically self-morphing structure through 4D printing. Virtual Phys. Prototyp. 2016, 12, 61–68, doi:10.1080/17452759.2016.1272174.
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Lee, S.-J.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–591, doi:10.1016/j.mattod.2017.06.005.
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D Printing Via Active Composite Materials. Available online: http://advances.sciencemag.org/ (accessed on 10 October 2020).
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171, doi:10.1016/j.jddst.2017.06.008.
- Sun, L.; Huang, W.M. Thermo/moisture responsive shape-memory polymer for possible surgery/operation inside living cells in future. Mater. Des. 2010, 31, 2684–2689, doi:10.1016/j.matdes.2009.11.036.
- Morouço, P.; Lattanzi, W.; Alves, N. Four-Dimensional Bioprinting as a new Era for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2017, 5, 61, doi:10.3389/fbioe.2017.00061.
- Introduction to 3D Printing—Additive Processes|Make. Available online: https://make.3dexperience.3ds.com/processes/introduction-to-additive-processes (accessed on 6 December 2020).
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 1–22, doi:10.1007/s11095-018-2454-x.
- Boydston, A.J.; Cao, B.; Nelson, A.; Ono, R.J.; Saha, A.; Schwartz, J.J.; Thrasher, C.J. Additive manufacturing with stimuli-responsive materials. J. Mater. Chem. A 2018, 6, 20621–20645, doi:10.1039/C8TA07716A.
- Senatov, F.; Zadorozhnyy, M.; Niaza, K.; Medvedev, V.; Kaloshkin, S.; Anisimova, N.; Kiselevskiy, M.; Senatov, F. Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur. Polym. J. 2017, 93, 222–231, doi:10.1016/j.eurpolymj.2017.06.011.
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130, doi:10.1016/j.biomaterials.2010.04.050.
- Vehse, M.; Petersen, S.E.; Sternberg, K.; Schmitz, K.-P.; Seitz, H. Drug Delivery from Poly (ethylene glycol) Diacrylate Scaffolds Produced by DLC Based Micro-Stereolithography. Macromol. Symp. 2014, 346, 43–47, doi:10.1002/masy.201400060.
- Bertsch, A.; Renaud, P. Microstereolithography. In Three-Dimensional Microfabrication Using Two-Photon Polymerization, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–56.
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001, doi:10.1088/1758-5090/ab6034.
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Wei, J.; Su, P.-C. 4D printing of high performance shape memory polymer using stereolithography. Mater. Des. 2017, 126, 219–225, doi:10.1016/j.matdes.2017.04.049.
- Saxena, P.; Bissacco, G.; Meinert, K. Ælkær; Danielak, A.H.; Ribó, M.M.; Pedersen, D.B. Soft tooling process chain for the manufacturing of micro-functional features on molds used for molding of paper bottles. J. Manuf. Process. 2020, 54, 129–137, doi:10.1016/j.jmapro.2020.03.008.
- Arcaute, K.; Mann, B.; Wicker, R. Acta Biomaterialia Stereolithography of spatially controlled multi-material bioactive poly (ethylene glycol) scaffolds. Acta Biomater. 2010, 6, 1047–1054, doi:10.1016/j.actbio.2009.08.017.
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur. Polym. J. 2018, 101, 169–176, doi:10.1016/j.eurpolymj.2018.02.023.
- Gorji, N.E.; Saxena, P.; Corfield, M.; Clare, A.; Rueff, J.-P.; Bogan, J.; González, P.G.; Snelgrove, M.; Hughes, G.; O’Connor, R.; et al. A new method for assessing the recyclability of powders within Powder Bed Fusion process. Mater. Charact. 2020, 161, 110167, doi:10.1016/j.matchar.2020.110167.
- Gurung, D. Technological Comparison of 3D and 4D Printing. 2017. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C23&as_ylo=2016&q=Technological+comparison+of+3D+and+4D+printing+Dilip+Gurung&btnG=%0Ahttps://www.theseus.fi/bitstream/handle/10024/130325/Thesis_Dilip.pdf?sequence=1 (accessed on10 September 2020).
- Ji, S.; Guvendiren, M. Recent Advances in Bioink Design for 3D Bioprinting of Tissues and Organs. Front. Bioeng. Biotechnol. 2017, 5, 23, doi:10.3389/fbioe.2017.00023.
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92, doi:10.1016/j.ijpharm.2014.09.044.
- Shishkovsky, I.; Scherbakov, V. 4D manufacturing of intermetallic SMA fabricated by SLM process. Laser 3D Manuf. V 2018, 10523, 1052311, doi:10.1117/12.2288176.
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172, doi:10.3389/fbioe.2018.00172.
- Kashyap, D.; Kumar, P.K.; Kanagaraj, S. 4D printed porous radiopaque shape memory polyurethane for endovascular embolization. Addit. Manuf. 2018, 24, 687–695, doi:10.1016/j.addma.2018.04.009.
- Bodaghi, M.; Damanpack, A.R.; Liao, W.-H. Triple shape memory polymers by 4D printing. Smart Mater. Struct. 2018, 27, 065010, doi:10.1088/1361-665X/aabc2a.
- Ge, Q.; Dunn, C.K.; Qi, H.J.; Dunn, M.L. Active origami by 4D printing. Smart Mater. Struct. 2014, 23, doi:10.1088/0964-1726/23/9/094007.
