Insulin is used for the treatment of diabetes mellitus, which is characterized by hyperglycemia. Subcutaneous injections are the standard mode of delivery for insulin therapy; however, this procedure is very often invasive, which hinders patient compliance, particularly for individuals requiring insulin doses four times a day. Furthermore, cases have been reported of sudden hypoglycemia occurrences following multidose insulin injections. Such an invasive and intensive approach motivates the quest for alternative, more user-friendly insulin administration approaches. For example, transdermal delivery has numerous advantages, such as prolonged drug release, low variability in the drug plasma level, and improved patient compliance.
- Insulin
- Skin Route
- Diabetes Mellitus
1. Introduction
In the last few decades, diabetes mellitus has emerged universally as an epidemic, and has become the fifth most prominent cause of mortality [1].
More than 422 million individuals worldwide have diabetes, according to a WHO report (see: https://www.who.int/health-topics/diabetes#tab=tab_1). This number could increase to 693 million by 2045 if proper actions are not taken [2][3]. In general, there are two classes of diabetes mellitus, i.e., type 1 and type 2. Type 1 is mostly due to a total insulin deficiency, but the causes of type 2 are varying degrees of insulin resistance, impaired insulin secretion, and elevated glucose production. Type 1 may be further subcategorized into type 1A diabetes mellitus, i.e., the autoimmune degradation of β-cells, and type 1B, i.e., idiopathic insulin deficiency [4]. The incidence of diabetes is growing owing to an aging population and improved diagnosis [5][6].
The development of insulin has been identified as one of the most significant events in the treatment of diabetes. The production of human insulin analogs using recombinant technology was seen as a huge step forward [7]. Insulin therapy has a significant role in treating type 1 diabetes. The subcutaneous route has been the most widely used, as it precludes enzymatic insulin degradation in the digestive tract. Healthy glycemic controls need to be preserved in type 1 diabetes, requiring at least three or maybe more daily insulin shots. Nevertheless, this route comes with the risk of infection and inflammation induced by the use of subcutaneous needles. Later, alternative routes—for instance, pulmonary, nasal, and oral routes—were investigated [8][9]. Pens, jet injectors, sharp needles, supersonic injectors, and infusion pumps have been introduced to minimize pain and enhance adherence to insulin regimens [10][11][12][13]. Still, compared to subcutaneous injections, the insulin absorption from the aforementioned techniques into the blood is quite low, and, consequently, other systems for insulin therapy are required [14][15]. Some noninvasive methods are being explored for insulin delivery [14]. Recently, there has been significant interest in the delivery of drugs via the transdermal route [16]. The transdermal route is an interesting choice for insulin delivery, as this approach would mitigate the pain and infection risk related to subcutaneous injections [17]. Furthermore, the transdermal route ensure patient compliance as well as delivery-controlled insulin release over time [18][19]. Still, the transdermal delivery of drugs is restricted, owing to the low permeability of the stratum corneum [20][21][22][23][24]. In recent years, a number of experimental techniques seeking to improve transdermal insulin delivery have been proposed [18][25][26][27][28][29][30]. In this review, different transdermal insulin delivery techniques and their improvements for diabetes care are highlighted. A schematic illustration of various strategies for insulin delivery via the transdermal route is presented in Figure 1.
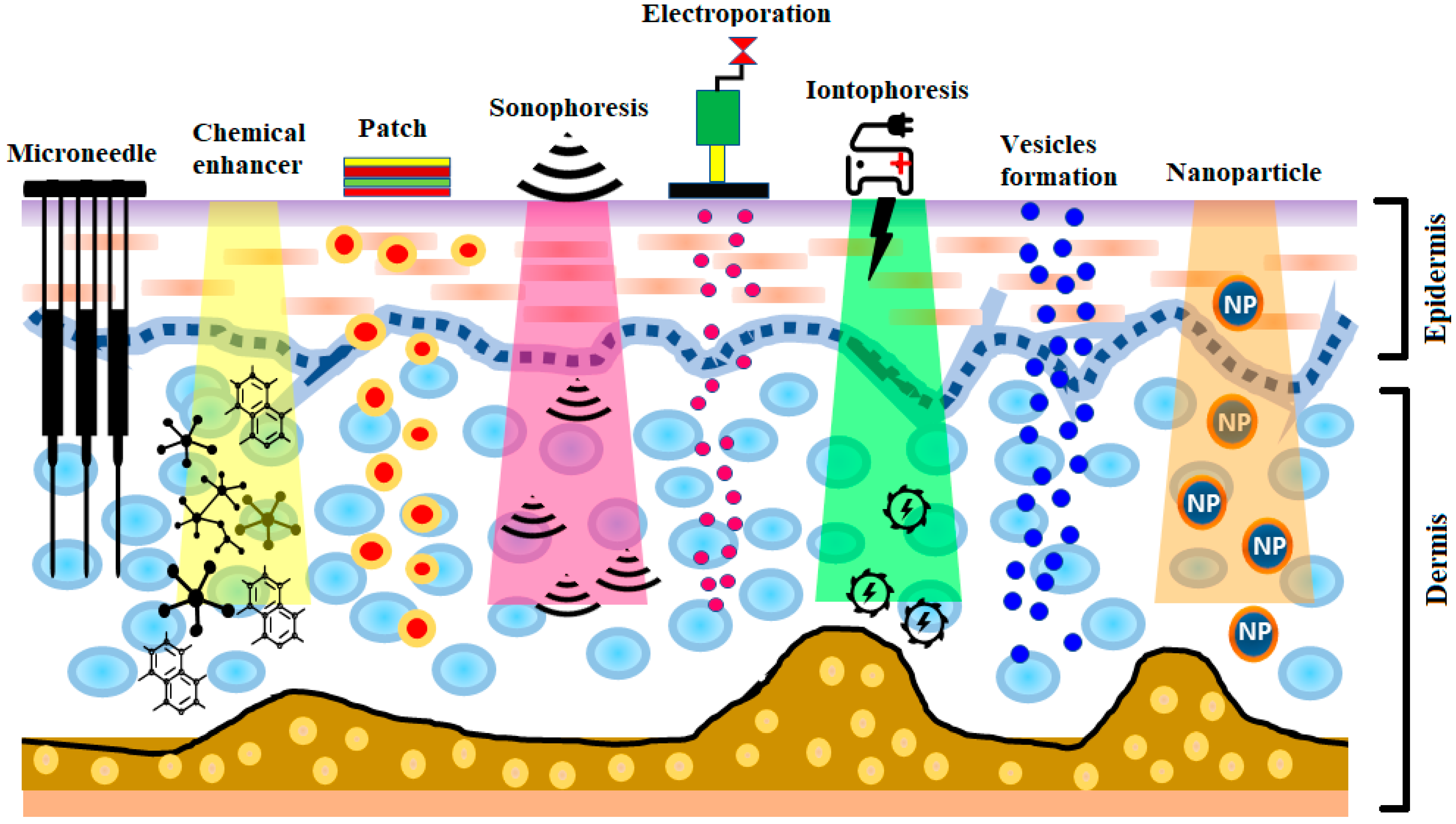
2. Delivery of Insulin via Skin Route
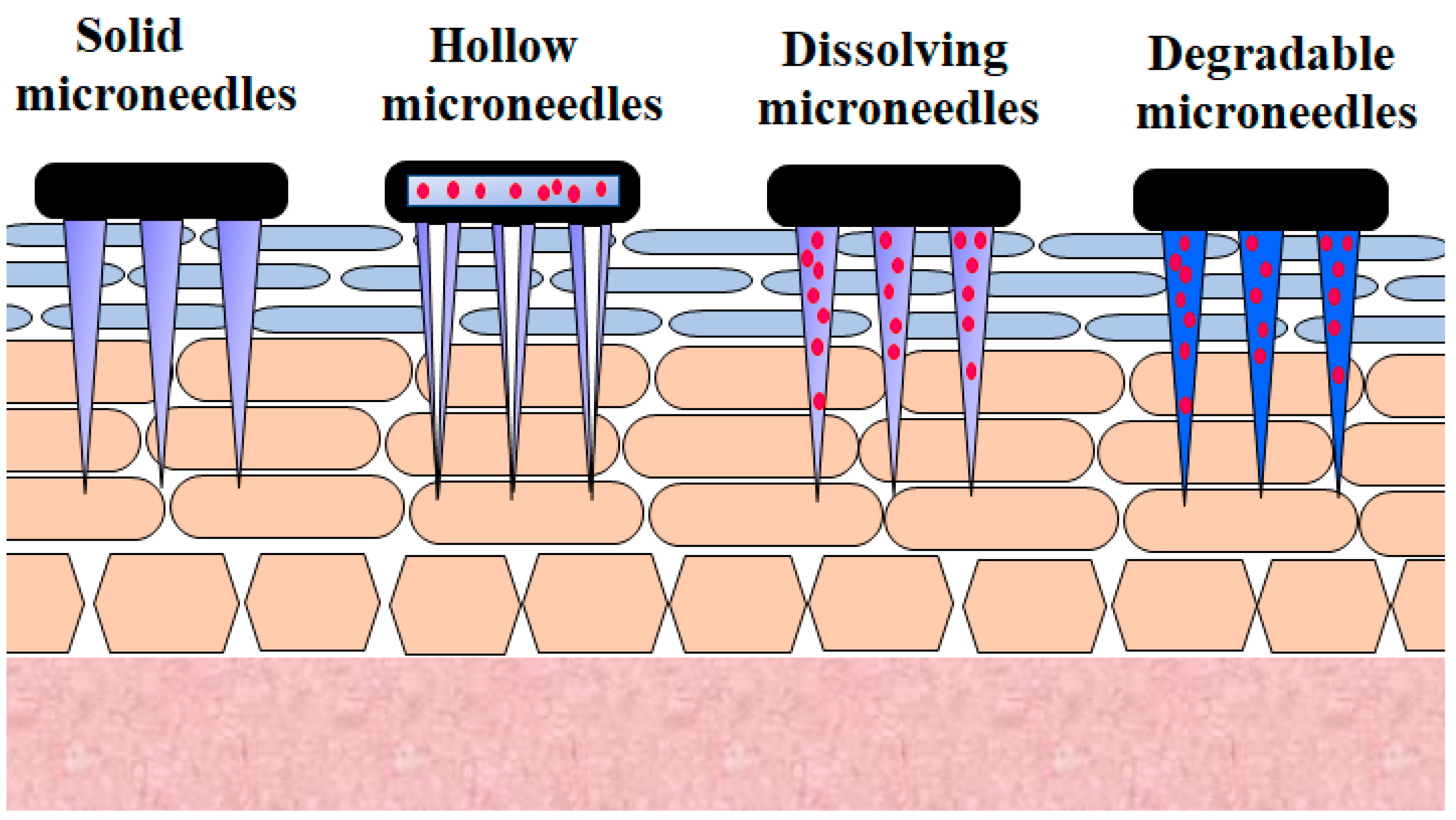
2.1. Microneedle
Microneedle technology offers an appealing technique for generating reversible skin microchannels that improves the skin permeability and allows the delivery of a wide variety of biotherapeutics, including insulin [31]. The delivery of microneedles at the site of application causes substantially less anxiety, pain, and tissue harm, owing to their minute size, as opposed to that of the 26-G hypodermic needle [32]. Micrometer-sized needles are sufficiently long to reach the corneum [33][34][35][36]. They are adequately narrow and sharp to cause minimal trauma and decrease the probability of infection [32][37]. This method offers a similar efficiency to standard injections. Besides this, the microneedle approach reduces the inherent problems associated with other invasive techniques [38]. Various microneedles employed for the transdermal delivery of insulin are presented in Figure 2.
2.2. Chemical Permeation Enhancer
The term “chemical permeation enhancer” applies to a substance or mixture of substances that enhances the permeability of the skin. Several groups of permeation enhancers have been tested for the delivery of various lipophilic and hydrophilic drugs(s) using both human and animal skins [39][40].
Transdermal enhancers (linolenic acid and oleic acid) and microwave techniques have recently been investigated to improve transdermal insulin permeation. The transdermal enhancer, linoleic acid, was the least active in terms of increasing the delivery of transdermal insulin, while the permeability enhancer, oleic acid, was found to be stronger than linolenic acid but failed to provide significant insulin permeation. The best result was found with microwave technology that facilitates insulin absorption and decreases the blood glucose levels in animals [41].
Previously, different chemical permeation enhancers were examined by Yerramsetty et al. to ameliorate skin permeability for the delivery of insulin. Amongst the investigated permeation enhancers, a total of eight, i.e., decanol, menthone, oleic acid, cycloundecanone, cis-4-hexen-1-ol, 2,4,6-collidine, octaldehyde, and 4-octanone, were found to be highly enhancing and nontoxic, five (cis-4-hexen-1-ol, 2,4,6-collidine, cycloundecanone, 4-octanone, and octaldehyde) of which were new discoveries [42].
2.3. Patches
In particular, transdermal patches are an appealing dosage method for the predictable and consistent delivery of insulin into the bloodstream. Insulin patches contribute to patient-friendly, noninvasive and painless delivery of insulin. Even, in the case of hyperinsulinemia, patients can easily remove the patches. Recently, nanoheaters incorporated into insulin patches were shown to effectively release insulin, and demonstrated comparable in vivo activity in mice with respect to s.c. injection of insulin [43].
In 2002, an innovative transdermal lipid-based system (Biphasix-insulin) was produced by King et al. and tested for blood glucose-reducing efficacy in a diabetic rat model induced by streptozotocin. Biphasix-insulin-containing transdermal patches (recombinant human insulin dose 10 mg) were administered to abdominal skin of rats with diabetes for 48 h. A blood glucose level decrease of 43.7%, compared to initial values, was observed. Further, the insulin bioavailability was improved by 21.5%, based on the serum insulin noted from the transdermal Biphasix-insulin patches. It was concluded that the Biphasix device successfully administered insulin via the skin route. These findings support the use of patches containing insulin for human use [29]. In 2003, King et al. reported that insulin in biphasic vesicle-containing transdermal patches had been administered to the abdominal skin of diabetic rats for 73 h, and the levels of blood glucose tested using a glucose meter every 2–10 h. ELISA was used to measure the inguinal lymph node insulin samples. The findings showed that insulin increased in the lymph nodes in a manner that depended on the dose and time. The maximum transdermal lymph node insulin concentrations were reported at 73 h with both 140 IU and 280 IU doses of recombinant insulin. The authors concluded that lymph transport is involved after transdermal insulin delivery of biphasic vesicles [44].
2.4. Sonophoresis
The use of ultrasound to enhance transdermal drug transport is referred to as phonophoresis or sonophoresis (Figure 3) [45]. It was observed that the increase in insulin delivery by the skin route that resulted from the use of ultrasound waves (low-frequency 20–100 kHz) could be due to the disruption of stratum corneum layers [46][47]. While sonophoresis has received considerable attention from researchers, its mechanisms are not fully understood, although several probable mechanisms have been suggested, the most plausible of which is cavitation [48][49].
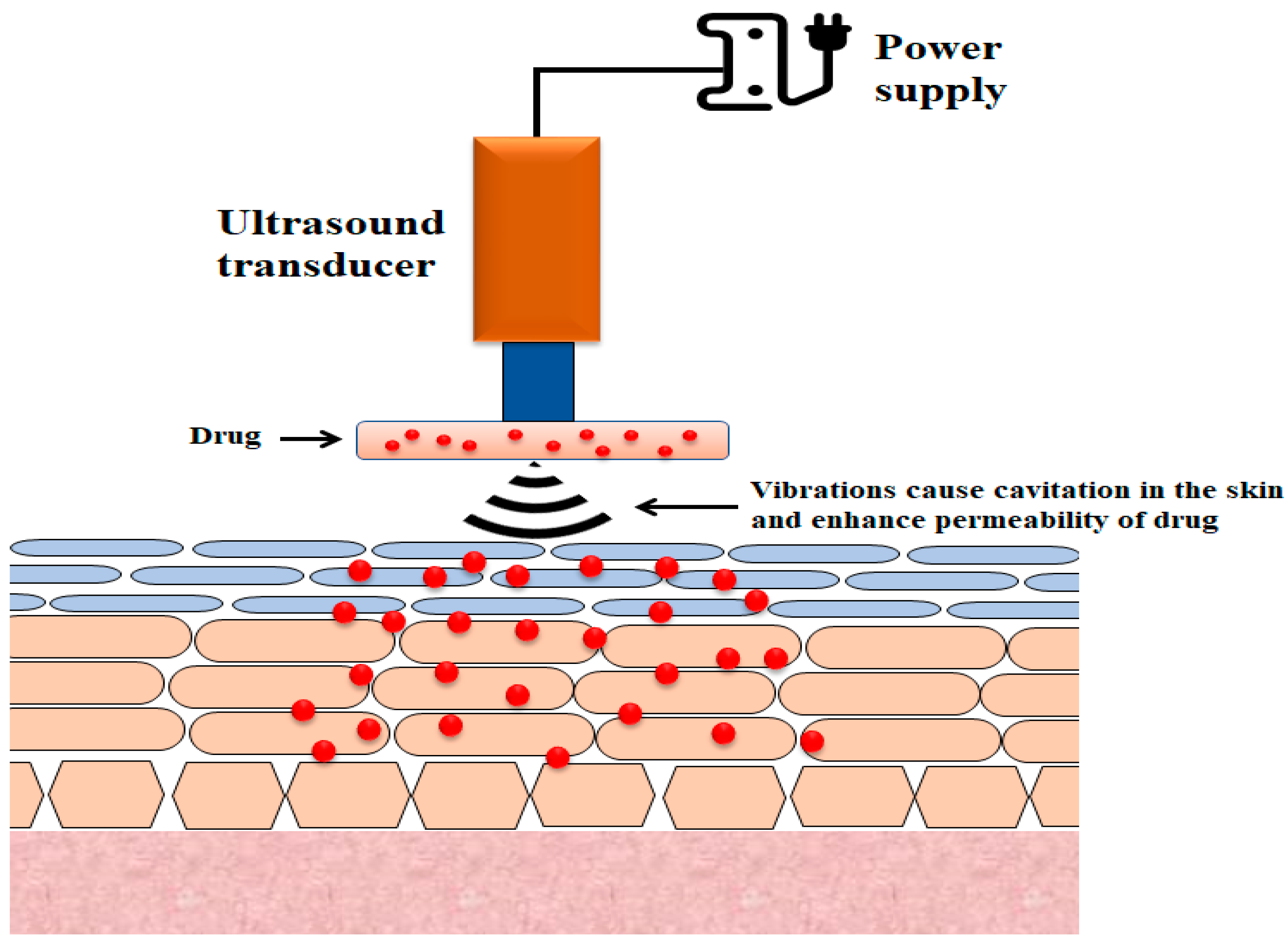
2.5. Electroporation
In this process, short, high-voltage electric field signals generate transient aqueous paths in the stratum corneum [50][51][52][53]. Researchers showed that electroporative pulses could be used in diabetic rabbits to regulate blood sugar by improving insulin transportation through the skin. It was highlighted that the increment in insulin dose and electroporative pulses, and decrease in the field strength of electroporation, contributed to a dramatic reduction in the blood sugar levels [54]. In the original report, investigators used a combination of electroporation and iontophoresis to study insulin permeation in rats. The investigators found that the combination of these techniques led to an increase in insulin plasma levels in comparison to those reported following electroporation [55]. In another study, an in vivo potency of the electroporation of insulin as a solution, insulin solution (s.c.), nanoparticle (i.v.) and nanoparticles (electroporation) was discussed. These findings indicated that polymeric nanosystem electroporation was an attractive substitute to injectable administration [56]. Other research indicated that, compared to electroporation alone, electroosmosis combined with electroporation in the presence of 1,2-dimyristoylphophatidylserine (a saturated anionic lipid) contributed a substantially higher transport rate of insulin [57].
2.6. Iontophoresis
One possible method for the improvement of drug delivery is transdermal iontophoresis [58][59]. Hao et al. stated that transdermal insulin delivery could potentially be achieved by combining iontophoresis and some enhancers [60]. Elsewhere, transdermal insulin delivery through porcine epidermis was observed by combining the use of iontophoresis with different chemical enhancers [61][62]. In 2002, researchers demonstrated the application of simultaneous techniques, such as iontophoresis + electroporation, for insulin permeation augmentation through human cadaver skin ex vivo [63]. Transdermal insulin delivery using the technique of iontophoresis was demonstrated in [26][64][65][66][67][68]. In a prior study, pretreatment (wiping) of skin using alcohol before iontophoresis was said to produce an impressive increment in insulin transdermal transport [69].
2.7. Vesicular Formulations
Liposomes are widely-studied nano-sized lipid vesicles that could be beneficial in the delivery of drugs via the dermal or transdermal routes. Liposomes, as drug carriers, offer many advantages that are reported elsewhere [70][71][72].
Techniques such as using combinations of two or more enhancers or the liposomal formula of insulin were investigated by Ogiso et al. The highest blood sugar lowering action that continued up to 10 h was exhibited by a transdermal system comprising liposomes insulin, d-limonene, and taurocholate. A high hypoglycemia effect was also achieved with a blend of n-octyl-beta-d-thioglucoside, cineol, and deoxycholate, or d-limonene and n-octyl-beta-d-thioglucoside. The authors clearly showed that the absorption of insulin in the stratum corneum of rat skin could be achieved under certain circumstances [73].
2.8. Microemulsion
Microemulsions typically droplets of less than 100 nm in size, and are thermodynamically stable clear liquids [74][75][76]. Microemulsions have been extensively studied and have gained considerable interest as vehicles of transdermal administration [77][78][79][80][81][40].
In 2013, insulin emulgel was prepared using emu oil (composed of fatty acids obtained from emu, a bird, Dromaius Novae-Hollandiae, native to Australia) as a permeation enhancer. The biological activity of emulgel alone and in combination with iontophoresis was tested using albino rabbits. The authors claimed that the optimized formulation [F4: emu oil (7.5% w/w) and polysorbate 80 (5.0% w/w)] showed a maximum insulin permeation flux of 4.88 μg/cm2/h through rat skin. A pharmacodynamic study indicated that the blood glucose level decreased from an initial value of 250 mg/dL to 185 mg/dL and the initial value to 125 mg/dL in 2 h in the group treated with insulin emulgel alone and insulin emulgel + iontophoresis respectively [45].
Transdermal microemulsions containing insulin were formulated with 10% oleic acid, 50% surfactant phase, and 2% DMSO, providing a maximum flux of 4.93 μg/cm2/h across goat skin. The authors concluded that there was considerable potential to use microemulsions for insulin delivery via the skin route [46].
2.9. Nanoparticles
Earlier, the feasibility of the use of transdermal insulin nanoparticles by means of a supercritical antisolvent micronisation procedure was investigated. The authors indicated that the supercritical antisolvent procedure provided uniform spherical insulin nanoparticles of particle sizes 68.2 ± 10.8 nm. The research indicated that the supercritical antisolvent process did not cause insulin degradation. An in vitro evaluation revealed that the insulin nanoparticles followed Fick’s first diffusion law, and displayed a good permeation rate. The authors found that the prepared nanoparticles containing insulin may have promising possibilities for the transdermal delivery of diabetes chemotherapy [82].
2.10. Microdermabrasion
Microdermabrasion has been previously used to minimize the presence of wrinkles, scars, and fine lines [83][84][85]. Previously, this approach was adopted as a tool to mitigate the hindering nature of the stratum corneum [86][87][88][89].
The application of microdermabrasion to improve skin insulin permeability was investigated by Andrews et al., who highlighted that microdermabrasion could improve the permeability of the skin to insulin at levels that are adequate to stabilize the range of blood glucose in rats with diabetes [90].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13010100
References
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int. J. Health Sci. (Qassim) 2007, 1, V–VIII.
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790–14801.
- Zhao, R.; Lu, Z.; Yang, J.; Zhang, L.; Li, Y.; Zhang, X. Drug Delivery System in the Treatment of Diabetes Mellitus. Front. Bioeng. Biotechnol. 2020, 8, 880.
- Rai, V.K.; Mishra, N.; Agrawal, A.K.; Jain, S.; Yadav, N.P. Novel drug delivery system: An immense hope for diabetics. Drug Deliv. 2016, 23, 2371–2390.
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843.
- Cheng, Y.J.; Kanaya, A.M.; Araneta, M.R.G.; Saydah, S.H.; Kahn, H.S.; Gregg, E.W.; Fujimoto, W.Y.; Imperatore, G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019, 322, 2389–2398.
- Rosenfeld, L. Insulin: Discovery and controversy. Clin. Chem. 2002, 48, 2270–2288.
- Verma, A.; Kumar, N.; Malviya, R.; Sharma, P.K. Emerging Trends in Noninvasive Insulin Delivery. J. Pharm. (Cairo) 2014, 2014, 378048.
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629.
- Al-Tabakha, M.M.; Arida, A.I. Recent challenges in insulin delivery systems: A review. Indian J. Pharm. Sci. 2008, 70, 278–286.
- Kesavadev, J.; Saboo, B.; Krishna, M.B.; Krishnan, G. Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas. Diabetes Ther. 2020, 11, 1251–1269.
- Selam, J.L. Evolution of diabetes insulin delivery devices. J. Diabetes Sci. Technol. 2010, 4, 505–513.
- Penfornis, A.; Personeni, E.; Borot, S. Evolution of devices in diabetes management. Diabetes Technol. Ther. 2011, 13, S93–S102.
- Yaturu, S. Insulin therapies: Current and future trends at dawn. World J. Diabetes 2013, 4, 1–7.
- Gradel, A.K.J.; Porsgaard, T.; Lykkesfeldt, J.; Seested, T.; Gram-Nielsen, S.; Kristensen, N.R.; Refsgaard, H.H.F. Factors Affecting the Absorption of Subcutaneously Administered Insulin: Effect on Variability. J. Diabetes Res. 2018, 2018, 1205121.
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215.
- El Khafagy, S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546.
- Ng, L.C.; Gupta, M. Transdermal drug delivery systems in diabetes management: A review. Asian J. Pharm. Sci. 2020, 15, 13–25.
- Hadebe, S.I.; Ngubane, P.S.; Serumula, M.R.; Musabayane, C.T. Transdermal delivery of insulin by amidated pectin hydrogel matrix patch in streptozotocin-induced diabetic rats: Effects on some selected metabolic parameters. PLoS ONE 2014, 9, e101461.
- Antunes, E.; Cavaco-Paulo, A. Stratum corneum lipid matrix with unusual packing: A molecular dynamics study. Colloids Surf. B Biointerfaces 2020, 190, 110928.
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855.
- Fernandez-Garcia, R.; Lalatsa, A.; Statts, L.; Bolas-Fernandez, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817.
- Mitragotri, S.; Farrell, J.; Tang, H.; Terahara, T.; Kost, J.; Langer, R. Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J. Control. Release 2000, 63, 41–52.
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268.
- Sen, A.; Daly, M.E.; Hui, S.W. Transdermal insulin delivery using lipid enhanced electroporation. Biochim. Biophys. Acta 2002, 1564, 5–8.
- Pillai, O.; Borkute, S.D.; Sivaprasad, N.; Panchagnula, R. Transdermal iontophoresis of insulin. II. Physicochemical considerations. Int. J. Pharm. 2003, 254, 271–280.
- Sintov, A.C.; Wormser, U. Topical iodine facilitates transdermal delivery of insulin. J. Control. Release 2007, 118, 185–188.
- Cevc, G.; Gebauer, D.; Stieber, J.; Schatzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta 1998, 1368, 201–215.
- King, M.J.; Badea, I.; Solomon, J.; Kumar, P.; Gaspar, K.J.; Foldvari, M. Transdermal delivery of insulin from a novel biphasic lipid system in diabetic rats. Diabetes Technol. Ther. 2002, 4, 479–488.
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70.
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137.
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594.
- Sivamani, R.K.; Stoeber, B.; Wu, G.C.; Zhai, H.; Liepmann, D.; Maibach, H. Clinical microneedle injection of methyl nicotinate: Stratum corneum penetration. Skin Res. Technol. 2005, 11, 152–156.
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760.
- Coulman, S.A.; Anstey, A.; Gateley, C.; Morrissey, A.; McLoughlin, P.; Allender, C.; Birchall, J.C. Microneedle mediated delivery of nanoparticles into human skin. Int. J. Pharm. 2009, 366, 190–200.
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113.
- Kaushik, S.; Hord, A.H.; Denson, D.D.; McAllister, D.V.; Smitra, S.; Allen, M.G.; Prausnitz, M.R. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001, 92, 502–504.
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587.
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155.
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal delivery enhancement of carvacrol from Origanum vulgare L. essential oil by microemulsion. Int. J. Pharm. 2020, 579, 119052.
- Harjoh, N.; Wong, T.W.; Caramella, C. Transdermal insulin delivery with microwave and fatty acids as permeation enhancers. Int. J. Pharm. 2020, 584, 119416.
- Yerramsetty, K.M.; Rachakonda, V.K.; Neely, B.J.; Madihally, S.V.; Gasem, K.A. Effect of different enhancers on the transdermal permeation of insulin analog. Int. J. Pharm. 2010, 398, 83–92.
- Pagneux, Q.; Ye, R.; Chengnan, L.; Barras, A.; Hennuyer, N.; Staels, B.; Caina, D.; Osses, J.I.A.; Abderrahmani, A.; Plaisance, V.; et al. Electrothermal patches driving the transdermal delivery of insulin. Nanoscale Horiz. 2020, 5, 663–670.
- King, M.J.; Michel, D.; Foldvari, M. Evidence for lymphatic transport of insulin by topically applied biphasic vesicles. J. Pharm. Pharmacol. 2003, 55, 1339–1344.
- Akram, M.; Naqvi, S.B.; Khan, A. Design and development of insulin emulgel formulation for transdermal drug delivery and its evaluation. Pak. J. Pharm. Sci. 2013, 26, 323–332.
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Development and evaluation of microemulsions for transdermal delivery of insulin. ISRN Pharm. 2011, 2011, 780150.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102–111.
- Biju, S.S.; Talegaonkar, S.; Mishra, P.R.; Khar, R.K. Vesicular systems: An overview. Indian J. Pharm. Sci. 2006, 68, 141–153.
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474.
- Marwah, H.; Garg, T.; Rath, G.; Goyal, A.K. Development of transferosomal gel for trans-dermal delivery of insulin using iodine complex. Drug Deliv. 2016, 23, 1636–1644.
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012, 20, 355–363.
- Date, A.A.; Patravale, V.B. Microemulsions: Applications in transdermal and dermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 547–596.
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494.
- Mohammad, E.A.; Elshemey, W.M.; Elsayed, A.A.; Abd-Elghany, A.A. Electroporation Parameters for Successful Transdermal Delivery of Insulin. Am. J. Ther. 2016, 23, e1560–e1567.
- Tokumoto, S.; Higo, N.; Sugibayashi, K. Effect of electroporation and pH on the iontophoretic transdermal delivery of human insulin. Int. J. Pharm. 2006, 326, 13–19.
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol. Physiol. 2008, 21, 246–259.
- Murthy, S.N.; Zhao, Y.L.; Marlan, K.; Hui, S.W.; Kazim, A.L.; Sen, A. Lipid and electroosmosis enhanced transdermal delivery of insulin by electroporation. J. Pharm. Sci. 2006, 95, 2041–2050.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289.
- Lauterbach, A.; Muller-Goymann, C.C. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163.
- Hao, J.S.; Zheng, J.M.; Yang, W.Z. Transdermal iontophoresis of insulin: Effect of penetration enhancers on blood glucose level in diabetic rats. Yao Xue Xue Bao 1995, 30, 776–780.
- Rastogi, R.; Anand, S.; Dinda, A.K.; Koul, V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev. Ind. Pharm. 2010, 36, 993–1004.
- Rastogi, S.K.; Singh, J. Effect of chemical penetration enhancer and iontophoresis on the in vitro percutaneous absorption enhancement of insulin through porcine epidermis. Pharm. Dev. Technol. 2005, 10, 97–104.
- Pan, Y.; Zhao, H.Y.; Zheng, J.M. The enhancing effect of electroporation and iontophoresis on the permeation of insulin through human skin. Yao Xue Xue Bao 2002, 37, 649–652.
- Pillai, O.; Panchagnula, R. Transdermal iontophoresis of insulin. V. Effect of terpenes. J. Control. Release 2003, 88, 287–296.
- Pillai, O.; Kumar, N.; Dey, C.S.; Borkute, S.; Nagalingam, S.; Panchagnula, R. Transdermal iontophoresis of insulin. Part 1: A study on the issues associated with the use of platinum electrodes on rat skin. J. Pharm. Pharmacol. 2003, 55, 1505–1513.
- Pillai, O.; Kumar, N.; Dey, C.S.; Borkute; Sivaprasad, N.; Panchagnula, R. Transdermal iontophoresis of insulin: III. Influence of electronic parameters. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 399–408.
- Pillai, O.; Nair, V.; Panchagnula, R. Transdermal iontophoresis of insulin: IV. Influence of chemical enhancers. Int. J. Pharm. 2004, 269, 109–120.
- Pillai, O.; Panchagnula, R. Transdermal iontophoresis of insulin. VI. Influence of pretreatment with fatty acids on permeation across rat skin. Skin Pharmacol. Physiol. 2004, 17, 289–297.
- Langkjaer, L.; Brange, J.; Grodsky, G.M.; Guy, R.H. Iontophoresis of monomeric insulin analogues in vitro: Effects of insulin charge and skin pretreatment. J. Control. Release 1998, 51, 47–56.
- Li, Y.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Liposomes modified with bio-substances for cancer treatment. Biomater. Sci. 2020, 23, 6442–6468.
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122.
- Pace, J.R.; Jog, R.; Burgess, D.J.; Hadden, M.K. Formulation and evaluation of itraconazole liposomes for Hedgehog pathway inhibition. J. Liposome Res. 2020, 30, 305–311.
- Ogiso, T.; Nishioka, S.; Iwaki, M. Dissociation of insulin oligomers and enhancement of percutaneous absorption of insulin. Biol. Pharm. Bull. 1996, 19, 1049–1054.
- Peltola, S.; Saarinen-Savolainen, P.; Kiesvaara, J.; Suhonen, T.M.; Urtti, A. Microemulsions for topical delivery of estradiol. Int. J. Pharm. 2003, 254, 99–107.
- El Maghraby, G.M. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: Effect of phase transition. Colloids Surf. B Biointerfaces 2010, 75, 595–600.
- Sintov, A.C.; Botner, S. Transdermal drug delivery using microemulsion and aqueous systems: Influence of skin storage conditions on the in vitro permeability of diclofenac from aqueous vehicle systems. Int. J. Pharm. 2006, 311, 55–62.
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292.
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—A review. Int. J. Pharm. 2020, 578, 119127.
- Russell-Jones, G.; Himes, R. Water-in-oil microemulsions for effective transdermal delivery of proteins. Expert Opin. Drug Deliv. 2011, 8, 537–546.
- Ferreira, P.G.; Noronha, L.; Teixeira, R.; Vieira, I.; Borba-Santos, L.P.; Vicosa, A.; de Moraes, M.; Calil-Elias, S.; de Freitas, Z.; da Silva, F.C.; et al. Investigation of a Microemulsion Containing Clotrimazole and Itraconazole for Transdermal Delivery for the Treatment of Sporotrichosis. J. Pharm. Sci. 2020, 109, 1026–1034.
- Zhang, Y.; Cao, Y.; Meng, X.; Li, C.; Wang, H.; Zhang, S. Enhancement of transdermal delivery of artemisinin using microemulsion vehicle based on ionic liquid and lidocaine ibuprofen. Colloids Surf. B Biointerfaces 2020, 189, 110886.
- Zhao, X.; Zu, Y.; Zu, S.; Wang, D.; Zhang, Y.; Zu, B. Insulin nanoparticles for transdermal delivery: Preparation and physicochemical characterization and in vitro evaluation. Drug Dev. Ind. Pharm. 2010, 36, 1177–1185.
- Bhalla, M.; Thami, G.P. Microdermabrasion: Reappraisal and brief review of literature. Dermatol. Surg. 2006, 32, 809–814.
- Fujimoto, T.; Shirakami, K.; Tojo, K. Effect of microdermabrasion on barrier capacity of stratum corneum. Chem. Pharm. Bull. (Tokyo) 2005, 53, 1014–1016.
- Freedman, B.M.; Rueda-Pedraza, E.; Waddell, S.P. The epidermal and dermal changes associated with microdermabrasion. Dermatol. Surg. 2001, 27, 1031–1034.
- Lee, W.R.; Shen, S.C.; Kuo-Hsien, W.; Hu, C.H.; Fang, J.Y. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J. Investig. Dermatol. 2003, 121, 1118–1125.
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Fang, Y.P.; Hu, C.H. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: A comparison with iontophoresis and electroporation. Br. J. Dermatol. 2004, 151, 132–140.
- Lee, W.R.; Tsai, R.Y.; Fang, C.L.; Liu, C.J.; Hu, C.H.; Fang, J.Y. Microdermabrasion as a novel tool to enhance drug delivery via the skin: An animal study. Dermatol. Surg. 2006, 32, 1013–1022.
- Gill, H.S.; Andrews, S.N.; Sakthivel, S.K.; Fedanov, A.; Williams, I.R.; Garber, D.A.; Priddy, F.H.; Yellin, S.; Feinberg, M.B.; Staprans, S.I.; et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur. J. Pharm. Sci. 2009, 38, 95–103.
- Andrews, S.; Lee, J.W.; Choi, S.O.; Prausnitz, M.R. Transdermal insulin delivery using microdermabrasion. Pharm. Res. 2011, 28, 2110–2118.
