Accumulating evidence shows that oxidative stress plays an essential role in the pathogenesis and progression of many diseases. The imbalance between the production of reactive oxygen species (ROS) and the antioxidant systems has been extensively studied in pulmonary, neurodegenerative and cardiovascular disorders; however, its contribution is still debated in gastrointestinal disorders. Evidence suggests that oxidative stress affects gastrointestinal motility in obesity, and post-infectious disorders by favoring the smooth muscle phenotypic switch toward a synthetic phenotype. Here is to gain insight into the role played by oxidative stress in gas-trointestinal pathologies (GIT), and the involvement of ROS in the signaling underlying the mus-cular alterations of the gastrointestinal tract (GIT).
- oxidative stress
- gastrointestinal diseases
- gastrointestinal muscle inflammation
- antioxidants
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Oxidative stress in living organisms results from the imbalance between the production of reactive oxygen species (ROS) and the ability to neutralize them. The disparity between excessive reactive molecules and weak endogenous defense leads to damage to cell structures and molecules such as lipids, proteins, and DNA, ultimately contributing to the pathogenesis of a wide range of diseases. ROS, when available in appropriate low amounts, act as signal transduction molecules driving cell activities and also provide cell protection [1]. On the other hand, if generated in excess, as in inflammation, ROS can trigger the production of additional highly reactive species [2]. Crucially is the oxidative modification of key enzymes or regulatory sites, whose redox modification triggers cell signaling alteration and programmed cell death. Oxidative stress and inflammation are closely linked. Oxidative stress can cause inflammation and this, in turn, induces oxidative stress generating a vicious circle [3,4] that results in cell damage, which promotes a pro-inflammatory environment [5].
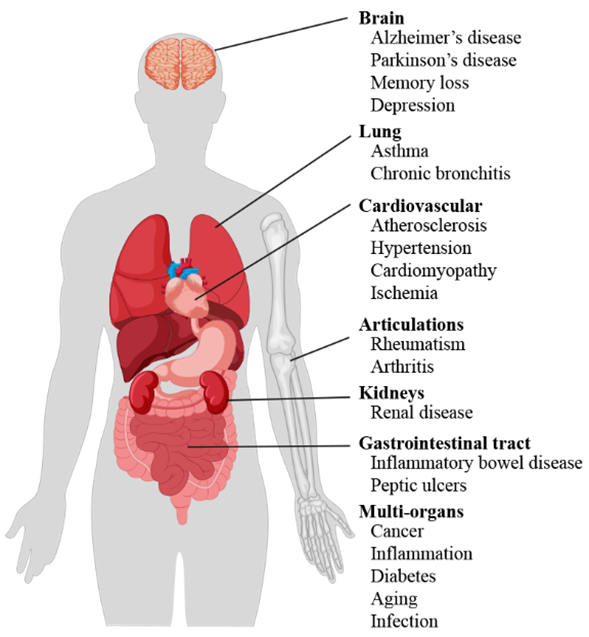
Literature data confirm the key role of oxidative stress in etiology of numerous and different diseases (Figure 1), including metabolic syndrome [6], atherosclerosis [7], cardiovascular disease [8,9], cancer [10,11], neurodegenerative disorders [12,13] diabetes [14], infertility [15], renal diseases [16], and gastrointestinal and hepatic diseases [17].
Figure 1. Scheme of oxidative stress-induced diseases in humans.
Being involved in the absorption of nutrients and in the immune response, the gastrointestinal tract (GIT) plays a key role also in the production of ROS. Several evidences highlight how the pathogenesis of various GIT diseases, including colorectal and gastric cancers [18–20], inflammatory bowel disease (IBD) [21,22], and peptic ulcers [23], is due, at least in part, to oxidative stress.
The GIT tissue is structured into four layers: the mucosa (epithelium, lamina propria, and muscular mucosae), the submucosa, the muscularis propria (inner circular muscle layer, intermuscular space, and outer longitudinal muscle layer), and the serosa.
The intestinal epithelia are exposed continuously to a wide variety of potentially harmful substances and act as a selective barrier between the tissues and luminal environment of the GIT. There are several stressors, which induce the generation of free radicals and result in oxidative stress and GIT inflammatory responses involving the epithelium and immune/inflammatory cells [24]. Although there is enough information on the role played by oxidative stress in the damage of intestinal mucosa, little is known about the involvement of the surrounding muscle layers.
Knowledge of the biochemical mechanisms underlying the alterations induced by oxidative stress at the GIT level, as well as of the physiological responses of the different GIT layers to such stress, is mandatory to better understand either pathogenesis of GIT diseases or to develop new and more effective therapeutic strategies.
This review summarizes the current understanding of the role of oxidative stress in GIT pathophysiology, also discussing the specific molecular mechanisms involved, focusing particular attention on the implication of the muscular layers of the GIT.
2. Oxidative Stress
Oxidative stress occurs when, in tissues and organs, the formation of highly reactive molecules e.g., ROS, reactive nitrogen species (RNS), and reactive sulfur species (RSS), overcome the endogenous antioxidant defense system capacities, leading to cellular damage and dysfunctions that result in a wide range of diseases. The reactive species are constantly generated within cells at low concentrations as a result of normal metabolic processes. They can also results from the exposure to external factors like radiation (X-rays and UV), ozone, air pollutants, cigarette smoke, bacteria, viruses, drugs, etc. [25], or as the outcome of an acute or chronic cellular stress. The reactive species can be free radicals and non-radical oxidants. The free radicals are unstable because of unpaired electrons presence in their outer electron orbit. Since free radicals are highly unstable and reactive, tend to neutralize themselves by reacting with other molecules causing their oxidation [26]. Therefore, by reacting with important biological molecules, including DNA, lipids and proteins, they can cause damage on various levels [27]. Proteins, being among the main components of the cells, represent major targets for free radicals [28]. Free radicals can induce some protein modifications, i.e., unfolding or alteration of protein structure, most of which, fortunately, are essentially harmless events [29]. While the reversible oxidative changes are involved in the regulation of protein activity, irreversible protein changes can lead to their inactivation with consequent lasting harmful cellular effects [29].
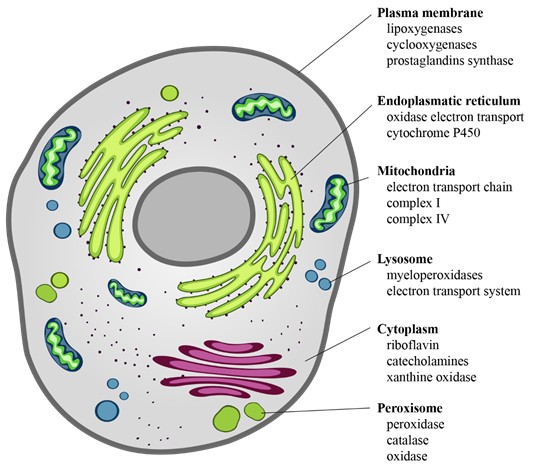
The intracellular sources of chemical reactive species are mainly mitochondria, endoplasmic reticulum, lysosomes, peroxisomes, cytosol, and plasma membrane [30] (Figure 2). ROS derive from the chemical reduction of molecular oxygen and, among the main ones, we find: the free radicals, such as superoxide anion radical (O2·−), hydroxyl radical (·OH), as well as non-radical oxidant, such as hydrogen peroxide (H2O2) and hypochlorous acid (HClO) [31]. Among the RNS, the major players are peroxynitrite radical (ONOO−), ozone, and nitric oxide (·NO) [32]. The new identified RSS include thiol radical (RS), and RSS both formed by the reaction between ROS and thiols. Similarly, RSS include radical species, such as (RSR·), glutathionyl radical (GSSG·), and non-radicals ones, such as reactive sulfane species (RSR), reactive sulfur substances (SO2, SO3), etc. [33,34]. In particular, RSS are able to trigger both oxidation and reduction reactions with particular tropism for sulfur-containing molecules, such as peptides and proteins [33,34].
Figure 2. Cellular sources of ROS. ROS are the “by-products” of electron transfer reactions. The major source of ROS is the mitochondrial electron transport chain, followed by the NADPH oxidases present on either side of the plasma membrane. In the smooth endoplasmic reticulum, we find cytochrome P-450 and b5 families, which are responsible for a series of reactions to detoxify fat-soluble drugs and harmful metabolites. Peroxisomes, through their oxidases, are a significant source of total cellular H2O2 production. Moreover, they are responsible for dismutation of H2O2 to H2O and O2, and of fatty acids β-oxidation. Other enzymes, present free in the cytoplasm, such as xanthine oxidase, aldehyde oxidase, flavoprotein dehydrogenase, and tryptophan dioxygenase can produce ROS during catalytic cycling.
The most important sites of ROS production are the enzymes of the mitochondrial electron transport respiratory chain. Other enzymes catalyze chemical reactions contributing to the ROS formation, among them the homologs of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, phospholipase A2 (PLA2), uncoupled nitric oxide synthase (NOS) as well as cyclooxygenases (COX), xanthine oxidase (XO), lipoxygenases (LOXs), glucose oxidase, and myeloperoxidase (MPO) [24,35,36]. NADPH oxidase, an enzyme present in the plasma membrane, was initially discovered in the phagosomes of macrophages, neutrophils, and monocytes. There are six homologs of NADPH oxidase, NOX1, NOX3-5, and dual oxidase (DUOX) 1 and 2, with several intracellular localizations [37]. Literature data showed that NOX1 and DUOX2 have significant roles in Helicobacter pylori-induced gastric inflammation, inflammatory bowel disease (IBD), and tumors [24,38]. XO is present in the cytoplasm and also on the outer surface of the plasma membrane; it is mainly expressed in the liver and small intestinal mucosa within the gastrointestinal tract [39]. LOXs are non-heme iron enzymes that can generate ROS catalyzing oxidation of arachidonic acid (AA). MPO is a heme-enzyme localized in lysosomes of neutrophils, macrophages, and monocytes. Several data demonstrate that MPO activity is increased in inflamed mucosa in ulcerative colitis and also in H. pylori-infected subjects [24], playing a role in the development of H. pylori-induced atrophic gastritis. The chronic oxidative stress related to ulcerative colitis and H. pylori- infection could also lead to cancer, often associated with these diseases [40,41]. NOS is a heme-containing monooxygenase that generates NO. There are three different isoforms of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS), and endotoxin or cytotoxin-inducible NOS (iNOS) [42]. In GIT, NOS expression and activity are very important because the generation of NO maintains normal functions of mucosa and plays a cytoprotective role. Indeed, NO regulates blood flow, epithelial secretion, and barrier function of gastric mucosal [43] and represents one of the main enteric neurotransmitters mediating GI muscle relaxation [43,44]. However, NO can also have deleterious effects, and iNOS expression was found increased in chronic ulcerative colitis and peptic ulcer patients [24]. COX enzyme releases AA from the membrane phospholipids and catalyzes AA conversion to prostanoids. COX has two isoforms, COX-1 and COX-2, both of which are expressed in normal human gastric mucosa. COX-1 is constitutively expressed, while COX-2 is induced by inflammation and tumorigenesis [45]. COX-2 has also been reported to have cytoprotective functions in human colon and gastric cancer cells where it was induced during high osmotic stress [46]. Therefore, reactive oxygen species, including oxygen free radicals, are generated by the activity of several types of oxidases. Initially, O2 is reduced by the addition of electrons, thereby producing O2·− that can react with other endogenous molecules to generate secondary oxidizing molecules, such as ONOO-. Thereafter, the reduction of O2·− leads to the by-product H2O2 that is characterized by a long life span and relative stability. The latter is enzymatically converted into water and O2, or possibly into different metabolites, thus extinguishing the radical cascade [6].
Both O2·− and H2O2 are also important signaling molecules, particularly in vascular smooth muscle cells where they can trigger specific biochemical pathways that regulate the defense mechanisms following exposure to oxidative stress. At the center of these pathways are for example mitogen-activated protein kinases, and tyrosine kinases, and transcription factors [47]. Particularly transcription factors, such as activator protein-1 (AP-1), NF-κB, and/or NF-E2-related factor (NRF2) have been reported to also participate in redox-modulated cell signaling [48,49].
3. Antioxidants
If the body’s antioxidant defense system fails to neutralize the excess free radicals, the imbalance between oxidants and the defense system can lead to pathological conditions, including cancer [10,11], cardiovascular disease [7,8], neurodegenerative disorders [12,13], atherosclerosis [7], and others. Halliwell and Gutteridge defined antioxidants as “any substance that delays, prevents or removes oxidative damage to a target molecule” [50–52]. All living organisms are endowed with endogenous antioxidant defenses capable of contrasting and removing reactive chemical species. However, these defenses are insufficient to totally remove reactive species and completely prevent oxidative damage to cells, tissues, and organs [4]. The endogenous antioxidants can act at various levels: blocking the formation of radicals, neutralizing them by oxidizing themselves, or delaying the oxidation reactions of other molecules. Moreover, some antioxidants, acting as metal chelators, transform metal pro-oxidants into more stable chemical forms.
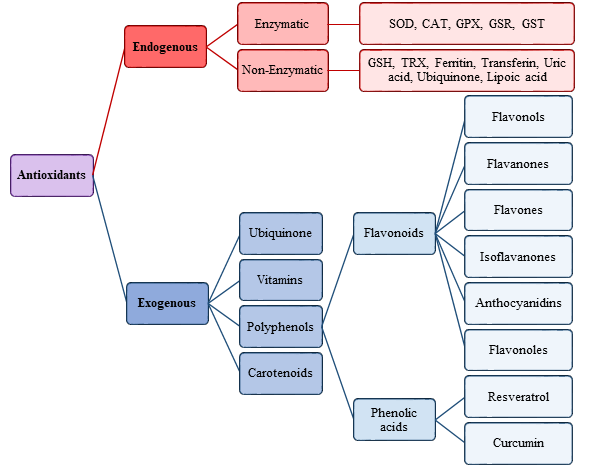
The antioxidants were be classified by Gutteridge and Halliwell into three categories: primary, secondary, and tertiary antioxidants, on the bases of their mechanism of action [51]. Primary antioxidants inhibit oxidant formation; secondary antioxidants function as scavengers of ROS, and tertiary antioxidants repair the oxidized molecules. Currently, antioxidants are substantially classified as enzymatic or non-enzymatic (Figure 3).
Figure 3. Scheme of endogenous and exogenous antioxidants. SOD, Superoxide dismutase; CAT, Catalase; GPX, Glutathione peroxidase; GSR, Glutathione reductase; GST, Glutathione transferase.
3.1. Enzymatic Antioxidants
Among the enzymatic antioxidants that contribute to the defense against the reactive species, we find catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), and glutathione reductase (GSR). Enzymatic antioxidants have both primary and secondary defense functions and represent an endogenous antioxidant system. Glutathione peroxidase, SOD, and catalase are the primary defense that prevents the formation or neutralize reactive species [53]. In particular, SOD and catalase provide major antioxidant defenses against ROS.
SOD catalyzes the dismutation of O2− into O2 and H2O2. In humans beings are present three isoforms of SOD [54]: cytosolic copper and zinc-containing enzyme (Cu-Zn-SOD), present in the mitochondrial inter-membranous space; manganese-requiring mitochondrial enzyme (Mn-SOD), present in the mitochondrial matrix; and extracellular Cu-Zn containing SOD (EC-SOD) [55]. H2O2 not scavenged by GPX located at the level of the mitochondrial matrix crosses the mitochondrial membrane towards the cytosol, where it can be scavenged either by cytosolic Cu-Zn-SOD or CAT [56]. Increased levels of all three SOD isoforms are present in intestinal tissues from IBD patients, particularly in the epithelium [57], and in patients with ulcer healing [58]. Increased expression of Mn-SOD is associated with colorectal cancer, and it was also found increased in normal mucosa of gastric adenocarcinoma as well as in squamous cell oesophageal carcinoma tissues [59]. Moreover, a gastrointestinal mucosal injury could be prevented by the presence of SOD [60].
CAT, present mainly in peroxisomes, dismutates H2O2 to H2O and O2 [61]. In humans, CAT has been found virtually in all organs although it is produced largely in liver, kidney, and erythrocytes. Lower catalase activity was observed in colorectal cancer [62], gastric adenocarcinoma, in H. pylori-infected stomach [62], and in Crohn’s disease [63].
GPX converts glutathione (GSH) into its oxidized form (GSSG), reduces H2O2 to H2O, and lipid hydroperoxides (ROOH) to the corresponding stable alcohols. The GPX reaction is paired to glutathione reductase (GSR), which maintains reduced glutathione (GSH) levels. GSR, GPX, and glutathione S-transferases (GST), form the glutathione system that in the GIT mucosa acts as an antioxidative barrier. This enzyme, generating GSH, is important for the protection of cell membranes, red blood cells, and hemoglobin to oxidative stress [64]. GPX is found in the mitochondria, cytoplasm, and extracellular space [65], and protects cells from the harmful consequences of peroxide decomposition. In humans, there are eight isotypes of GPX. While GPX1 is ubiquitous, GPX2 is specific for the gastrointestinal tract and protects the gut against the absorption of dietary hydroperoxides [66]. Moreover, GPX2 defends the gastrointestinal tract against ROS derived from gut inflammation associated with commensal bacteria [67]. Importantly, glucose-6-phosphate dehydrogenase, while not directly neutralizing the radicals, can be considered an antioxidant enzyme. This oxidoreductase maintains the level of NADPH, thus helping to keep glutathione in its reduced state (GSH) [68] and creating a reducing environment [53].
Thioredoxin reductase (TrxR) together with thioredoxin (Trx) forms the thioredoxin system. There are three TrxR isoforms: TrxR1 found in the cytoplasm, TrxR2 in mitochondria, and TrxR3 present only in specialized tissues. TrxR, by transferring reducing equivalents from NADPH to thioredoxin, keeps it in its reduced form [69]. It has been shown that a compensatory upregulation of TrxR mRNA in gastrointestinal cancer was induced by oxidative stress provoked by bile acids [70].
3.2. Non-enzymatic Antioxidants
Among the endogenous non-enzymatic antioxidants, there are glutathione and Trx. Glutathione is ubiquitously expressed mostly in its reduced form, GSH. Glutathione is a strong antioxidant, certainly one of the most important among those that the body can produce. Relevant is its action against both free radicals and molecules such as hydrogen peroxide, nitrites, nitrates, benzoates, and others. An important element for its functioning is NADPH. In fact, this molecule, a derivative of vitamin PP (nicotinic acid), functions as a redox cofactor of the enzyme GSR, which reduced glutathione (GSH) from oxidized glutathione (or GSSG) through electrons transferred from NADPH to GSSG [53].
Trx contains two free sulfhydryl groups of two cysteine residues. It is involved in the biosynthesis of deoxynucleotides, since it reduces the oxidized ribonucleotide reductase by yielding their hydrogens to the two oxidized sulfhydryl groups of the ribonucleotide reductase. Trx is present in the cytoplasm, membranes, and mitochondria but also in the extracellular space [71]. It showed a cytoprotective action in various inflammatory conditions, and was found to regulate the activity of redox-sensitive transcription factors, which are part of the antioxidant defence system.
Ubiquinone, also known as CoQ, is a lipophilic molecule existing in three different redox states: fully oxidized, partially reduced (ubisemiquinone), and fully reduced (ubiquinol) [72]. It is found in the plasma membrane and in several intracellular membrane including mitochondrial ones where it plays a key role in energy production and ROS generation. In its fully reduced form, CoQ is a potential antioxidant. Experimental studies have shown a protective role of ubiquinone against protein carbonylation and oxidative damage to DNA [73,74]. Furthermore, it is also been shown that ubiquinone can prevent peroxidative damage to membrane phospholipids [75] and regenerate other powerful antioxidants, such as α-tocopherol and ascorbate, by recycling them back to their reduced active forms, thus increasing resources cellular antioxidants [76]. These properties make ubiquinone suitable as a food supplement to improve cellular bioenergetics and to counteract some age-related diseases [76].
Activator protein-1 (AP-1), nuclear factor kappa B (NF-κB), and nuclear factor (erythroid-derived 2)-like 2 related factor (NRF2) are three transcription factors that have been reported to be involved in redox-modulated signaling pathways. Indeed, oxidative stress up-regulates NF-κB activity, and AP-1 and NRF2 activation depends on the environmental and/or intracellular redox state. Under normal conditions, NRF2 is found blocked in the cytosol by its inhibitor, KEAP1. Oxidative modification of KEAP1 and NRF2 phosphorylation result in the release of NRF2 from KEAP1 [77] and its translocation into the nucleus, where it binds with antioxidant response elements involved in activation of gene expression, thereby protecting cells from free radical damage. Therefore, NRF2, through its interaction with antioxidant response element (ARE), is able to modulate the expression of defensive genes coding detoxifying enzymes and antioxidant proteins [78].
3.3. Exogenous Antioxidants
Besides the endogenous enzymatic and non-enzymatic antioxidant defenses, other antioxidants are also utilized by the body, which must necessarily be introduced through diet and for this reason are defined exogenous. In addition to endogenous antioxidants, exogenous ones act through different mechanisms and in different cellular compartments. They are mainly free radical scavengers: they neutralize free radicals, repair oxidized membranes, and decrease reactive oxygen species production [79]. Among the exogenous antioxidants, we find: vitamins (A, C, E, and K), enzyme cofactors (Q10), nitrogen compounds (uric acid), minerals (zinc, Zn and selenium, Se), and polyphenols (flavonoids and phenolic acid) [80]. Metals such as manganese, zinc, copper, iron, and selenium up-regulate the catalytic activity of antioxidant enzymes [81]. It has been indicated that an inadequate dietary intake of these trace minerals may compromise the effectiveness of antioxidant defense mechanisms [82].
Exogenous antioxidants have generated a growing interest in preventing or reducing oxidative stress. In fact, many epidemiological researches have highlighted how the use of foods containing antioxidants and scavengers has a potential protective effect against the disorders caused by oxidative stress [83–87]. By increasing the body’s natural antioxidant defenses, or by supplementing with dietary antioxidants, various chronic diseases can be prevented or their progression can be slowed down. Natural antioxidants such as flavonoids, tannins, and polyphenols act by donating electrons to intermediate radicals and play a role in the inhibition of lipid peroxidation. For example, vitamin E, particularly its active form α-tocopherol, protects cells from lipid peroxidation and helps in the prevention of chronic diseases associated with oxidative stress [88,89].
The antioxidant phytochemicals contained in vegetables and fruits are considered a benefits to the health. Indeed, several studies demonstrated that they have antioxidant abilities both in vitro and in vivo [90,91]. Moreover, literature data highlighted that antioxidant phytochemicals can also have anti-inflammatory action [92]. In fact, natural compounds such as curcumin, resveratrol, and anthocyanins could reduce inflammation via inhibition of prostaglandin production, NF-κB activity, and specific oxidative enzymes, as well as by increasing anti-inflammatory cytokine (e.g., IL-10) or decreasing pro-inflammatory cytokine (i.e., IL-1β) production [93,94].
This entry is adapted from the peer-reviewed paper 10.3390/antiox10020201