Xanthone is an aromatic oxygenated heterocyclic molecule, with a dibenzo-γ-pirone scaffold, known as 9H-xanthen-9-one, with the molecular formula of C13H8O2.
- xanthones
- natural products
- syntesis
- biological activities
1. Xanthone: The Molecule
The numbering and designation of rings A and B come from the biosynthetic pathways for the compounds from higher plants, A-ring (carbons 1–4) being acetate-derived whereas the shikimic acid pathway gives B-ring (carbons 5–8); the other carbon atoms are numbered according IUPAC 2004 recommendations for structure elucidation purposes.
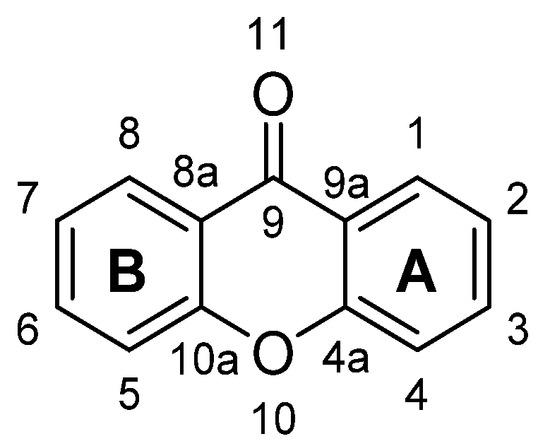
Figure 1. Structure of xanthone (9H-xanthen-9-one).
X-Ray diffraction data are an important tool, not only for structure elucidation but also to help in understanding the mechanism of action of the wide range of biological and pharmacological activities showed by xanthone derivatives. The crystal structure of 9H-xanthen-9-one (Figure 1) was first reported in 1982 and later, using more accurate experimental techniques, the data have been improved. Considering the molecule of xanthone itself in solid state, it is essentially planar due to the conjugated ring systems, except for the O(11) atom, which deviates 0.13 Å from the plane, with the central pyranoid ring with partial aromatic character. Due to the tricyclic-fused ring system, free rotation is limited. The rigidity of this scaffold contributes to the stability of the compound. For xanthone derivatives, slight differences can be found dependent on the nature of the substituents and their localization on the scaffold. The three-ring system can be slightly twisted along its longitudinal axis due to steric factors associated with the substituents, especially for bulky groups .
This work reviews the contributions of the corresponding author (M.M.M.P.) and her research group to Medicinal Chemistry concerning the isolation from plant and marine sources of xanthone derivatives as well as their synthesis, biological/pharmacological activities, formulation and analytical applications. Xanthone derivatives have a variety of activities with great potential for therapeutic applications due to their versatile framework. The group has contributed with several libraries of xanthones derivatives, with a variety of activities such as antitumor, anticoagulant, antiplatelet, anti-inflammatory, antimalarial, antimicrobial, hepatoprotective, antioxidant, and multidrug resistance reversal effects. Besides therapeutic applications, our group has also developed xanthone derivatives with analytical applications as chiral selectors for liquid chromatography and for maritime application as antifouling agents for marine paints.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26020431
