The development of engineered silica particles by using low-cost renewable or waste resources is a key example of sustainability. Rice husks have emerged as a renewable resource for the production of engineered silica particles as well as bioenergy.
- rice husk
- rice husk ash
- silica
1. Introduction
Global rice and paddy production in 2018 was approximately 996 million tons as reported by the Food and Agricultural Organization [1]. The countries with the largest volume of rice and paddy production are located in Asia (China, India, Indonesia, Bangladesh, Vietnam, and Thailand). Rice husk is a residue produced during the rice mill process and on average, accounts for 20% of the paddy produced. The rice husk output in 2018 was approximately 199 million tons. Many countries use rice husks as a renewable energy resource for power generation [2]. The heating value of the rice husk is 15 MJ kg−1, and there is an energy potential of 2985 PJ available per year [3]. Currently, rice husks are burnt in simple incinerators for resident energy, industrial streams, and thermal power plants in most Asian countries. Several Asian countries such as India, China, and Thailand are operating gasification power plants using rice husks [4][5]. Gasification power plants using rice husks have power capacities ranging between 20 and 400 kWe; however, they are still in the demonstration stage [6]. One ton of rice husk generates 800 kWh of electric power [7] and after generating electric power, approximately 0.195 tons of rice husk ash is produced as a byproduct. High ash content in rice husks causes operational problems and consequently renders their thermal conversion difficult and expensive. Therefore, the valorization of rice husk ash for value-added material applications is important for improving the economic return of the entire process. In addition, the extraction of inorganic compounds from rice husks before energy generation could be worthwhile to reduce the burden of the energy production process.
Rice husk ash mainly contains amorphous silica (SiO2) and other metallic impurities. Rice husk-derived silica has gained increasing interest as a renewable source. Engineered silica particles have recently been intensively studied for bio-applications [8][9][10], energy storage [11][12], bioremediation [13], and as construction materials [14][15][16][17]. By increasing the utilization of engineered silica particles, the synthesis of engineered silica from renewable resources is considered to enhance sustainability. The use of rice husk silica for synthesizing engineered silica particles has advantages not only in the economy but also in mitigating environmental issues [18].
2. Purified Silica Extraction from Rice Husk
2.1. Combustion to Remove Organic Contents
The most widely used method for obtaining silica from rice husks is direct combustion, resulting in the production of rice husk ash, which contains 85–95% silica [19][20][21]. The direct combustion of rice husk can produce thermal energy and can be used to generate steam, which subsequently drives the blades of a turbine to produce electricity. However, the direct combustion of rice husks generates greenhouse gases and emits significant quantities of particulate matter [22][23]. The emissions from rice husk burning contain CO2, CH4, CO, NOx, SO2, PM2.5, and PM10 of black carbon. Hence, when using direct combustion, it is important to always use both a dust collector and a gas absorber. The emission of CO2 from rice husk combustion is “net zero” because the hull reduces CO2 in nature. It also replaces the use of a fossil fuel. Therefore, the use of rice husk to produce energy is encouraged only when it is installed with emission control devices.
The phase change of silica depends on the combustion temperature. The silica in rice husks is a non-crystalline phase. As the combustion temperature increases over 600 °C, the phase transformation to tridymite and cristobalite starts [24]. However, the crystallization temperature varied depending on the chemical composition of rice husk ash [25]. It was also reported that pretreated rice husk remained amorphous up to 1000 °C. At higher combusting temperatures, the physical properties of silica also change. Zareihassangheshlaghi et al. compared the physical properties of rice husk ashes synthesized at 700 and 900 °C [26]. At a higher combustion temperature (900 °C), fewer metal impurities were observed. This might be due to the formation of more volatile and less stable phases that can be easily released into the gas phase. At this temperature, alkaline earth metal oxides can be refractory. At higher temperatures, the size of ash particles increases. Furthermore, the mesopores and micropores in the ash diminish after combustion at 900 °C. In rice husk ash, alkali metal impurities, such as K, P, Ca, and Mg, incorporate and form sticky alkali silicate at high combustion temperatures. This causes ash melting and agglomeration, which increases the size of particles and diminishes the meso- and micro-pores [27].
Previous studies report that combustion conditions could define the characteristics of silica; however, changes in characteristics could be within a narrow range: the specific surface area (11–39 m2 g−1), purity (29.7–96.7 wt%), and crystallinity (completely amorphous or partially crystalline) [25]. The characteristics of the shape, particle size, pore, and uniformity cannot be controlled by changing the combustion conditions. Therefore, chemical treatment should be followed to synthesize the engineered silica particles.
2.2. Principle of Chemically Extracting Silica from Rice Husk
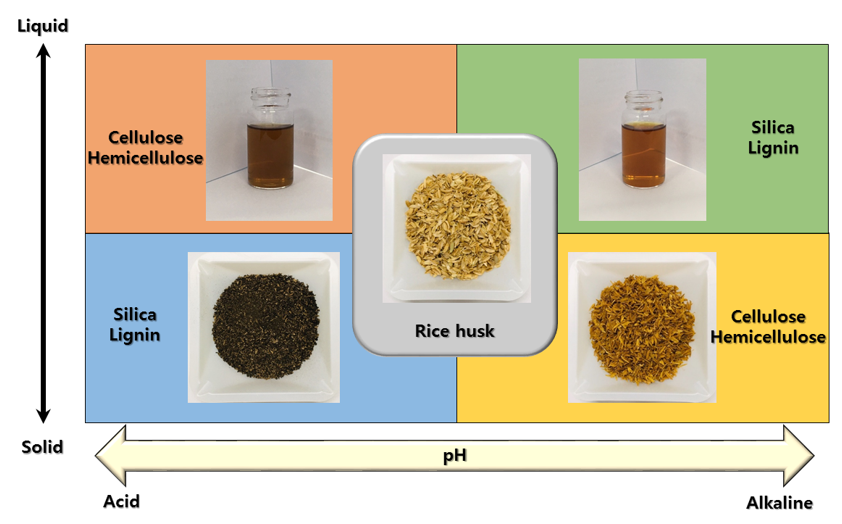
The main components of rice husk—cellulose, hemicellulose, lignin and inorganics—can be separated by their thermo-chemical properties. Figure 1 shows the phase changes of rice husk components depending on pH. Under acidic conditions, cellulose and hemicellulose are dissolved in the aqueous phase and can be separated from solid residues, mainly lignin and inorganics [28]. During the hydrolysis of polysaccharides, metallic impurities, such as K2O, P2O5, CaO, and MgO, were discarded by the washing step. Alkaline hydrothermal conditions induce the cleavage of ester linkages in lignin, which dissolves the lignin. After alkaline hydrothermal treatment, lignin can be removed from the solid residue [29][30]. The alkaline thermal treatment also dissolves silica and partially dissolves xylan to the liquid phase. The solubility of amorphous silica rapidly increases above pH 9.14 [31]. It can be again solidified by decreasing the pH. During solidification, silica particles can be engineered to have specific properties fit for their intended purpose. Residual organic compounds in the silica can be thermally removed at temperatures over 575 °C.
Figure 1. Phase change of rice husk components depending on pH.
2.3. Acid Leaching to Obtain High Purity Silica
Acid leaching to remove organic matter and metallic impurities from rice husks was conducted before or after the combustion process. Acid leaching can produce a higher purity and specific surface area when compared to without acid leaching [19][32][33][34]. Lee et al. compared the performances of three acid solutions—sulfuric acid, hydrogen chloride, and oxalic acid—to remove the organic matter and metallic impurities [19]. Sulfuric acid effectively dissolved and removed both cellulose and hemicellulose but was not effective in removing lignin. Both hydrogen chloride and oxalic acid mainly reduced hemicellulose. The removal of metallic impurities was investigated depending on the acid solutions. However, the capability of acid solutions to remove organic and metallic impurities in rice husk varied depending on the solution’s concentration, and the reaction time and temperature [35]. Traditionally, the acid leaching of rice husk was performed with sulfuric acid, hydrogen chloride [36], and nitric acid [33]. The use of a strong acid solution is significantly hazardous to the environment and human life. In addition, strong acid leaching produces not only soluble sugars but also smaller compounds such as aliphatic carboxylic acids and furans, which are inhibitors to microbes and enzymes. Therefore, attempts to replace these strong acid agents with environmentally harmless agents have been reported [19][37][38][39][40].
The environmentally harmless agents used to remove organic matter in rice husks were citric acid, ionic liquid, and deionized water. The use of citric acid could remove metallic impurities such as Na, K, Ca, Mg, Fe, Cu, etc., through a chelate reaction between carboxyl groups and metal elements [37][38]. Ionic liquids are green solvents and are effective in dissolving polysaccharides. The ionic liquids used to remove polysaccharides and metallic impurities from rice husks were 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium hydrogen sulfate. The dissolved cellulose can be recovered by mixing with distilled water and further used to produce fermentable sugar [41]. Trinh et al. reported that ionic liquid-treated cellulose significantly changed its crystallinity, surface morphology, and composition, resulting in improved enzymatic digestibility. Conventionally, lignocellulose in rice husks is simply burned to generate energy, which leads to air pollution. However, the use of ionic liquid-treated cellulose to produce biofuel does not produce any pollutants. Therefore, using ionic liquids improves both the purity of silica and the comprehensive utilization of lignocellulose. Shen et al. used deionized water to leach metallic impurities, but water leaching was effective only on the external surface [40]. Mochidzuki et al. performed water leaching under pressurized conditions using a batch autoclave and steam explosion [42]. Both autoclave hot-water and steam-explosion-treated silica showed improved purities, even comparable to those obtained with the hydrochloric acid treatment. The pressurized water treatment dissolved some portion of the silica in hot water and changed its structure, which is more applicable to the synthesis of water-glass-like materials.
Most studies reported that acid leaching could improve the amorphousness of silica [19][39][43]. Alkali metals in rice husks facilitate the initiation of the formation of cristobalite, which causes the phase transformation of the silica [44]. Acid leaching could remove alkaline metals, which prevent the phase transformation of the silica. In addition, Real et al. reported that the leaching of rice husks with an acid solution before their combustion would yield silica powder with a high specific surface area [32]. However, if the acid leaching was performed after combustion, the specific surface area of silica would be poor.
2.4. Alkali Extraction to Obtain Silicate
Silica can be extracted from rice husk by solubilizing it in an alkali solution and precipitating in an acidic medium. In general, sodium hydroxide was used to extract silica from the rice husk as sodium silicate. The following formula represents the chemical reaction:
|
SiO2 (s) + 2NaOH (l) → Na2SiO3 (l) + H2O (l) |
(1) |
Various hydrothermal conditions were used for the alkali extraction of silica. Most studies have prepared rice husk ash or acid-treated rice husk before the alkali extraction. Typically, thermal treatment is performed before alkali extraction to remove lignin. Otherwise, both lignin and silica were extracted from rice husks by alkali solution, which affected the quality of sodium silicate. In addition to the thermal treatment, organosolv fractionation can be applied to separate lignin by organic solvents such as ethanol [45] and 1,4-butanediol [46]. Rice husk ash was dissolved in sodium hydroxide solution at 80 °C [47], 90 °C [21][48], 100 °C [49], and even 150 °C [50]. In addition, a considerable amount of research has reported the performance of alkali extraction at room temperature [51][52][53][54][55]. In this case, rice husk ash was ground down to micron-sized particles and reacted for 24 h. The concentrations of sodium hydroxide were varied in the range of 1–10 M, which determined the reaction time. Bazargan et al. showed the removal of lignin and silica from the rice husk, not rice husk ash, by the assistance of sodium hydroxide and hydrogen peroxide [56]. However, recoveries of silica and lignin in alkaline peroxide solution were only 75 and 60%, respectively. Alkali extraction degrades lignin into phenolic compounds, such as benzoic acid, ferulic acid, and coniferyl aldehyde, and those cannot be utilized in further process.
This entry is adapted from the peer-reviewed paper 10.3390/su122410683
References
- Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 Nov. 2020).
- Bhattacharyya, C. Viability of off-grid electricity supply using rice husk: A case study from South Asia. Biomass Bioenergy 2014, 68, 44–54.
- Quispe, ; Navia, R.; Kahhat, R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Manag. 2017, 59, 200–210.
- Asadullah, Barriers of commercial power generation using biomass gasification gas: A review. Renew. Sustain Energy Rev. 2014, 29, 201–215.
- Hiloidhari, ; Baruah, D. Crop residue biomass for decentralized electrical power generation in rural areas (part 1): Investigation of spatial availability. Renew. Sustain Energy Rev. 2011, 15, 1885–1892.
- Assanee, ; Boonwan, C. State of the art of biomass gasification power plants in Thailand. Energy Procedia 2011, 9, 299–305.
- Pode, Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain Energy Rev. 2016, 53, 1468–1485.
- Lin, -S.; Hurley, K.R.; Haynes, C.L. Critical considerations in the biomedical use of mesoporous silica nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374.
- Liu, ; Li, C.; Cheng, Z.; Hou, Z.; Huang, S.; Lin, J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater. Sci. 2016, 4, 890–909.
- Wang, ; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327.
- Jung, S.; Ryou, M.-H.; Sung, Y.J.; Park, S.B.; Choi, J.W. Recycling rice husks for high-capacity lithium battery anodes. Proc. Natl. Acad. Sci. USA 2013, 110, 12229–12234.
- Shen, Rice husk silica-derived nanomaterials for battery applications: A literature review. J. Agric. Food Chem. 2017, 65, 995–1004.
- Kumari, ; Singh, D. A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol. Eng. 2016, 97, 98–105.
- Van Tuan, ; Ye, G.; Van Breugel, K.; Copuroglu, O. Hydration and microstructure of ultra high performance concrete incorporating rice husk ash. Cem. Concr. Res. 2011, 41, 1104–1111.
- Martirena, ; Monzó, J. Vegetable ashes as supplementary cementitious materials. Cem. Concr. Res. 2018, 114, 57–64.
- Kang, -H.; Hong, S.-G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2019, 115, 389–400.
- Kang, -H.; Kwon, Y.-H.; Hong, S.-G.; Chun, S.; Moon, J. Hydrated lime activation on byproducts for eco-friendly production of structural mortars. J. Clean Prod. 2019, 231, 1389–1398.
- Shen, Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain Energy Rev. 2017, 80, 453–466.
- Lee, H.; Kwon, J.H.; Lee, J.-W.; Lee, H.-s.; Chang, J.H.; Sang, B.-I. Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J. Ind. Eng. Chem. 2017, 50, 79–85.
- Fernandes, J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.; Rocha, T.L.; Brehm, F.A.; Modolo, R.C. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359.
- Zulfiqar, ; Subhani, T.; Husain, S.W. Towards tunable size of silica particles from rice husk. J. Non-Cryst. Solids 2015, 429, 61–69.
- Junpen, ; Pansuk, J.; Kamnoet, O.; Cheewaphongphan, P.; Garivait, S. Emission of air pollutants from rice residue open burning in Thailand, 2018. Atmosphere 2018, 9, 449.
- Lasko, ; Vadrevu, K. Improved rice residue burning emissions estimates: Accounting for practice-specific emission factors in air pollution assessments of Vietnam. Environ. Pollut. 2018, 236, 795–806.
- Nakata, ; Suzuki, M.; Okutani, T.; Kikuchi, M.; Akiyama, T. Preparation and properties of SiO2 from rice hulls. J. Ceram. Soc. Jpn. 1989, 97, 842–849.
- Beidaghy Dizaji, ; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre-and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083.
- Zareihassangheshlaghi, ; Beidaghy Dizaji, H.; Zeng, T.; Huth, P.; Ruf, T.; Denecke, R.; Enke, D. Behavior of metal impurities on surface and bulk of biogenic silica from rice husk combustion and the impact on ash-melting tendency. ACS Sustain. Chem. Eng. 2020, 8, 10369–10379.
- Alyosef, A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of biogenic silica generated by thermo chemical treatment of rice husk. Part. Sci. Technol. 2013, 31, 524–532.
- Pedersen, ; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. N. Biotechnol. 2010, 27, 739–750.
- Pedersen, ; Viksø-Nielsen, A.; Meyer, A.S. Monosaccharide yields and lignin removal from wheat straw in response to catalyst type and pH during mild thermal pretreatment. Process Biochem. 2010, 45, 1181–1186.
- Kristensen, B.; Thygesen, L.G.; Felby, C.; Jørgensen, H.; Elder, T. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5.
- Alexander, B.; Heston, W.; Iler, R.K. The solubility of amorphous silica in water. J. Phys. Chem. 1954, 58, 453–455.
- Real, ; Alcala, M.D.; Criado, J.M. Preparation of silica from rice husks. J. Am. Ceram. Soc. 1996, 79, 2012–2016.
- Liou, -H.; Yang, C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng. B 2011, 176, 521–529.
- Chen, ; Bie, H.; Bie, R. Leaching characteristics and kinetics of the metal impurities present in rice husk during pretreatment for the production of nanosilica particles. Korean J. Chem. Eng. 2018, 35, 1911–1918.
- Chakraverty, ; Mishra, P.; Banerjee, H. Investigation of combustion of raw and acid-leached rice husk for production of pure amorphous white silica. J. Mater. Sci. 1988, 23, 21–24.
- Vayghan, G.; Khaloo, A.; Rajabipour, F. The effects of a hydrochloric acid pre-treatment on the physicochemical properties and pozzolanic performance of rice husk ash. Cem. Concr. Compos. 2013, 39, 131–140.
- Umeda, ; Kondoh, K. High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J. Mater. Sci. 2008, 43, 7084–7090.
- Umeda, ; Kondoh, K. Process optimization to prepare high-purity amorphous silica from rice husks via citric acid leaching treatment. Trans. JWRI 2008, 37, 13–17.
- Chen, ; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of lignocellulose and synthesis of porous silica nanoparticles from rice husks: A comprehensive utilization of rice husk biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259.
- Shen, ; Liu, X.; Zhu, S.; Zhang, H.; Tan, J. Effects of calcination parameters on the silica phase of original and leached rice husk ash. Mater. Lett. 2011, 65, 1179–1183.
- Trinh, T.P.; Lee, Y.J.; Lee, J.-W.; Lee, H.-J. Characterization of ionic liquid pretreatment and the bioconversion of pretreated mixed softwood biomass. Biomass Bioenergy 2015, 81, 1–8.
- Mochidzuki, ; Sakoda, A.; Suzuki, M.; Izumi, J.; Tomonaga, N. Structural behavior of rice husk silica in pressurized hot-water treatment processes. Ind. Eng. Chem. Res. 2001, 40, 5705–5709.
- Ahmad Alyosef, ; Schneider, D.; Wassersleben, S.; Roggendorf, H.; Weiß, M.; Eilert, A.; Denecke, R.; Hartmann, I.; Enke, D. Meso/macroporous silica from miscanthus, cereal remnant pellets, and wheat straw. ACS Sustain. Chem. Eng. 2015, 3, 2012–2021.
- Moroz, K.; Maslennikova, G. Thermal transformations of silica. Glass Ceram. 1985, 42, 559–564.
- Kim, H.; Ryu, H.J.; Oh, K.K. Improvement of organosolv fractionation performance for rice husk through a low acid-catalyzation. Energies 2019, 12, 1800.
- Zhang, ; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73.
- Tchakouté, K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289.
- Geraldo, H.; Fernandes, L.F.; Camarini, G. Water treatment sludge and rice husk ash to sustainable geopolymer production. J. Clean. Prod. 2017, 149, 146–155.
- Tchakouté, K.; Rüscher, C.H.; Kong, S.; Ranjbar, N. Synthesis of sodium waterglass from white rice husk ash as an activator to produce metakaolin-based geopolymer cements. J. Build. Eng. 2016, 6, 252–261.
- Jahan, S.; Haris, F.; Rahman, M.M.; Samaddar, P.R.; Sutradhar, S. Potassium hydroxide pulping of rice straw in biorefinery initiatives. Bioresour. Technol. 2016, 219, 445–450.
- Bernal, A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Provis, J.L.; Delvasto, S. Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste Biomass Valoriz. 2012, 3, 99–108.
- Bernal, ; Rodríguez, E.; Mejía de Gutiérrez, R.; Provis, J.L. Performance at high temperature of alkali-activated slag pastes produced with silica fume and rice husk ash based activators. Mater. Constr. 2015, 65, e049.
- Kamseu, ; à Moungam, L.B.; Cannio, M.; Billong, N.; Chaysuwan, D.; Melo, U.C.; Leonelli, C. Substitution of sodium silicate with rice husk ash-NaOH solution in metakaolin based geopolymer cement concerning reduction in global warming. J. Clean. Prod. 2017, 142, 3050–3060.
- Mejía, ; de Gutiérrez, R.M.; Puertas, F. Rice husk ash as a source of silica in alkali-activated fly ash and granulated blast furnace slag systems. Mater. Constr. 2013, 63, 361–375.
- Mejía, M.; de Gutiérrez, R.M.; Montes, C. Rice husk ash and spent diatomaceous earth as a source of silica to fabricate a geopolymeric binary binder. J. Clean. Prod. 2016, 118, 133–139.
- Bazargan, ; Wang, Z.; Barford, J.P.; Saleem, J.; McKay, G. Optimization of the removal of lignin and silica from rice husks with alkaline peroxide. J. Clean. Prod. 2020, 260, 120848.