Inflammatory bowel disease (IBD) continue to cause substantial morbidity and massive productivity loss globally. IBD is more common among the productive generation (age group of <30yrs) and affects their quality of life. A single mechanism responsible for IBD is difficult to determine due to the complex interplay of multiple factors including host’s genetic predisposition and environmental factors. The relapsing nature of IBD demands repeated treatment implicating a substantial financial burden to individual patients and the concerned healthcare system, especially in developing nations. This review focuses on the causes of IBD,risk factors, current treatment options and challenges, the role played by the natural products in IBD health care; and situate these natural products within the current biodiscovery research agenda, including the applications of drug discovery techniques and the search for next generation drugs to treat IBD.
- IBD, causes, risk factors, treatments, drugs
The Hygiene Hypothesis is a central theme to the growing incidence of IBD, but it is still difficult to pinpoint which particular factors are responsible for causing IBD. Strachan first proposed the Hygiene Hypothesis in 1989 to explain the increasing incidence of atopy (allergic disorders) [55]. Later, many authors claimed through epidemiological studies and various experimental models that autoimmune disorders could be a result of broad environmental, infectious burden rather than individual behavior/hygiene [56–59]. According to the Rook’s reinterpretation of hygiene hypothesis (Old Friends Hypothesis as proposed in 2003), immunoregulatory disorders would occur first in those individuals with reduced/minimal contact with pathogens including commensal microbes and helminths (old friends) that are known to prime immunoregulation (Treg activity) in the human gut [60]. The rapid rise in the incidence of IBD over the last century in both developing and developed countries [3,15] could be related to improved hygiene practices such as access to clean water, non-contaminated food, and reduced family size [61]. While the definitive cause of IBD remains elusive and unknown, many studies point to 10 different causative factors (Figure 1) [23], with three major factors being genetic, environmental, and diet (which influences the host’s gut microbiota).
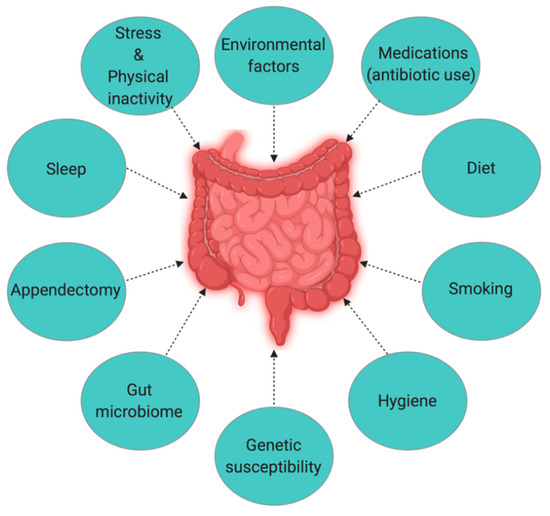
Figure 1. The interplay of factors causing inflammatory bowel disease (IBD).
Genetics
Genome-wide association studies identified 163 susceptible gene loci in IBD (with 110 common, 30 CD specific, and 23 UC specific) [62], highlighting the role of genetics in IBD pathogenesis. However, more than 50% of IBD susceptible gene loci are also associated with other inflammatory and autoimmune diseases [63]. MDR1 (multidrug resistance 1) gene in human chromosome 7 is one such gene that is associated with the pathogenesis of UC [54]. A comprehensive understanding of the mechanisms of IBD pathogenesis induced by genetic factors requires studying the role of an individual gene, which can be a challenging task. However, less than 50% concordance for IBD in twins represents the significant role that environmental factors might have in the development of IBD [64]. Another study conducted by Thompson et al. in British twins also reported similar (only 17% concordance for IBD) results among identical twins [65]. Moreover, the concordance between two disease conditions (CD and UC) among twins is not the same. Analysis of a Swedish twin cohort showed higher concordance (50%) for CD than for UC (18.8%), suggesting that genetic influence or heritability is higher in CD than in UC [64,66,67].
Environmental Factors
After extensive studies trying to determine the role of genetics in the pathogenesis of IBD have not yielded adequate evidence, many assume environmental factors to have a more significant role than genetics. Numerous studies have analyzed the linkage between various environmental factors and the development of IBD. When Khalili et al. studied the association of geographical variation with the incidence of IBD (both CD and UC) in two large prospective cohorts of US women (175,912), they found a higher incidence of both CD and UC among women residing in the northern latitudes, which could be attributable to less exposure to sunlight or ultraviolet B (UV-B) radiation [68]. UV-B radiation induces UV-Treg cells that have the potential to suppress inflammatory responses [69]. People living in higher altitudes are also more likely to have vitamin D deficiency due to insufficient exposure to sunlight [70]. Lack of vitamin D is also considered a possible cause of IBD since vitamin D receptor knock-out mice develop severe inflammation [71].
Numerous studies are suggesting a role for the environment in the pathogenesis of IBD, notably from a migration perspective [72–74]. For instance, children of immigrants who arrived in Canada at a younger age have an increased risk of IBD [75]. While Zoetendal et al. compared the risk of developing IBD among African-Americans and Africans inhabiting semi-urban (westernized) and rural environments respectively, the risk was significantly lower among those who were born and raised (first five years) in unhygienic environments like livestock farms compared to those living in the city [76]. Selective exposure to various environmental factors or conditions is also responsible for determining the microbiome composition. A study carried out in South Africa observed distinct gut microbiome composition in genetically similar populations in rural areas versus urban areas [77], where rural subjects contained significantly lesser Bacteriodetes populations than semi-urban and urban subjects. While a similar comparative study from India showed a dominance of phyla Bacteriodetes and Proteobacteria in the gut microbiome of rural subjects, phyla Firmicutes, and Lactobacillus in urban subject [78]. These findings indicate that exposure to different environmental conditions/lifestyles can influence the microbiome composition among a similar population.
Microbiota
The commensal human gut microbiota is essential for maintaining intestinal epithelial homeostasis and protection from mucosal injury [79]. The human lower GI tract has 1014 microbial cells [80], and Bacteriodetes and Firmicutes are the two most dominant phyla in the gut [81]. The gut microbiome determines the normal functioning of human health. For example, fibrinolytic bacteria degrade polysaccharides in the gut into smaller carbohydrates and short-chain fatty acids [82], and microbiota of the lower GI tract use dietary fiber as a source of energy [83]. Any changes in the composition of the healthy microbiota (dysbiosis) in the gut can trigger abnormal inflammatory responses. Firmicutes species, such as Faecalibacterium prausnitzii, has been reported to be poorly represented in patients with active IBD compared to healthy subjects [84]. The study conducted by Martin et al. further supports the protective role of this species, where intragastric administration of F. prausnitzii significantly decreased the severity of colitis in the trinitrobenzene sulfonic acid (TNBS)-induced mouse model of colitis [85]. The host must maintain a balance between recognizing pathogenic from commensal microbial species, as any disturbance in the composition of commensal species could trigger abnormal inflammatory responses such as IBD.
Diet and Smoking
Diet influences the composition of the microbiota and their metabolic activity in the human gut [86]. There is a growing concern that the western diet, rich in fats and sugars, is responsible for the change in the diversity and metabolic activity of human gut microbiota, thereby contributing to the increasing incidence of IBD [87,88]. The increase in the abundance of Bilophila wadsworthia due to an animal-based diet can facilitate the growth of microorganisms that can trigger IBD [87,88]. Moreover, B. wadsworthia also produces hydrogen sulfide that can cause damage to intestinal tissues [86]. Long-term dietary pattern influences the development of IBD [89]. For instance, the intake of fruits decreases the risks of developing CD [90], although the underlying mechanism is yet to be understood. Smoking is one of the contradictory factors linked to IBD. While smoking is harmful to CD patients, reports show beneficial in UC [63]. The positive effect of smoking in UC is evident from the “Boston Drugs Surveillance Program” [91], ”UC patients in Birmingham, England” [92], and ”Oxford Family Planning Association Contraceptive Study” [93]. Additionally, the transdermal treatment of active UC patients with nicotine patches also showed better remission compared to the placebo group [94]. However, it is still controversial, and more research is required to determine if nicotine is one of the active components of cigarette smoking that is responsible for the beneficial effects on the UC disease course.
Sleep Deprivation, Stress, and Physical Inactivity
Inadequacy of sleep and psychological distress are additional intrinsic factors known to associated with inflammation and the inflammation system. Sleep disturbances are said to be common in IBD patients [95,96]. Some studies [97,98] have reported that symptoms of depression and anxiety cause clinical recurrence in IBD patients. However, stressful life events are not associated with the onset of inflammatory disease [99]. Alteration of sleep pattern or circadian rhythms [100] and insufficient sleep (<6 h/day) [96] has a direct impact on disease course and severity. A study involving 136 Japanese IBD patients found sleep disturbances as a potential risk factor of disease flare-up for both UC and CD within one year [95], but a similar kind of study (3173 IBD patients with sleep disturbances) conducted by Ananthakrishnan et al. [101] could observe an increased risk of disease flares only in CD within 6 months. A positive correlation between psychological distress and IBD flare-ups [102,103] indicates the need for timely psychological therapy in IBD patients.
Appendectomy
Appendectomy (i.e., surgical removal of the appendix) and its association with the development of UC and CD is a scarcely explored area of research [104]. Few studies involving both humans as well as animal models showed evidence for a role of the appendix in gastroenterology. T-cell receptor-α mutant mice (TCR-α−/−) appendectomized at a young age (3–5 weeks old) contained more mesenteric lymph node (MLN) cells compared to the placebo group (sham-operated TCR- α−/− mice), indicating that the appendix could be an important site for priming MLN cells involved in causing IBD [105]. Similarly, Mombaerts et al. also found that an increase in the number of MLN cells in TCR-α−/− mice is related to the development of IBD [106]. A few studies and case reports have also shown the positive effect of appendectomy on the clinical course of UC in human subjects. A study of IBD patients in Australia confirmed that appendectomy before diagnosis delays disease onset of both UC and CD and results in fewer flare-ups in the case of UC when compared with patients without prior appendectomy [107]. A case report from Korea also confirmed that a patient with UC experienced a more extended period of remission after appendectomy [108]. However, the therapeutic relationship between CD and appendectomy remains inconclusive. [30].
Antibiotic Use
A leading hypothesis in the etiology of IBD is the alteration in the human gut microbiota that triggers abnormal inflammatory responses, including IBD. Multiple factors are assumed to be responsible for inducing gut dysbiosis. Childhood exposure to antibiotics is one among them [109]. Children exposed to antibiotics at an early stage [109–112] and adults who had medication for acute gastroenteritis [113] possess higher risks for IBD. The frequency of use of antibiotics and the age at the time of use may have a varying effect as risks for IBD tend to decrease with increasing age at the time of exposure [114]. Regular intake of non-steroidal anti-inflammatory drugs like aspirin showed a strong positive correlation with only CD [115].
This entry is adapted from the peer-reviewed paper 10.3390/jcm9051273
