Tissue Chips (TCs) and Microphysiological Systems (MPSs) that incorporate human cells are novel platforms to model disease and screen drugs and provide an alternative to traditional animal studies. This review highlights the basic definitions of TCs and MPSs, examines four major organs/tissues, identifies critical parameters for organization and function (tissue organization, blood flow, and physical stresses), reviews current microfluidic approaches to recreate tissues, and discusses current shortcomings and future directions for the development and application of these technologies. The organs emphasized are those involved in the metabolism or excretion of drugs (hepatic and renal systems) and organs sensitive to drug toxicity (cardiovascular system). This article examines the microfluidic/microfabrication approaches for each organ individually and identifies specific examples of TCs. This review will provide an excellent starting point for understanding, designing, and constructing novel TCs for possible integration within MPS.
- tissue chips
- microphysiological systems
- microfluidics
- organ-on-a-chip
- tissue-on-a-chip
- body-on-a-chip
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Background
For decades, our understanding of essential cellular functions and how they relate to human health has relied on model systems that investigate the molecular basis of normal physiology and pathophysiology. Cellular-level models provide a high degree of biological specificity that permits the study of specific cellular signaling pathways in the absence of noise or crosstalk due to interactions among other tissues and organs. However, the physiological relevance of cellular-level models is low, as they fail to recreate the systemic interactions seen in whole organisms. For this reason, while the identification of therapeutic targets typically begins at the cellular level, drugs require subsequent pre-clinical validation in higher-level animal models before progressing to human studies. The cost and time associated with obtaining regulatory approval following pre-clinical studies and clinical trials for a single drug are upwards of USD 2.55 billion and between 10–15 years, respectively [1–3]. A significant contributor to this expensive and time-consuming drug discovery pathway is the reliance on animal models, which oftentimes are not accurate predictors of the efficacy and toxicity of drugs in humans. Nearly 30% of drugs deemed safe in animal studies are toxic to humans, and around 60% of drugs that are effective in animals provide no discernible benefit to humans [4]. These discrepancies are due, in large part, to interspecies differences in the activities of drug-metabolizing enzymes. Thus, there is a critical need for alternatives to animal models that closely replicate human physiology to predict the efficacy, safety, bioavailability, and toxicity of candidate therapeutics.
A potential solution for increasing the predictive power of pre-clinical studies is the use of in vitro model systems constructed with human cells. However, such model systems’ clinical relevance relies on their ability to accurately mimic human physiology and function. Breakthroughs in developmental and stem cell biology have resulted in the availability of human induced pluripotent stem cells (hiPSCs), representing renewable and patient-specific source of cells for constructing complex, multicellular in vitro models. Recent advancements in tissue engineering, biomaterial science, three-dimensional (3D) fabrication techniques, and microfluidic technologies have enabled new methods for creating 3D tissue constructs or tissue chips (TCs). TCs, also frequently referred to as organ chips (OCs), replicate essential aspects of human organ structure and function, and their development has received strong support from federal funding agencies. In 2010, the National Institutes of Health (NIH) Common Fund, in collaboration with the Food and Drug Administration (FDA), started the Regulatory Science Program to make medical product development and evaluation more efficient. As part of this program, the first TC project, which sought to develop a heart-lung model to test the safety and efficacy of drugs, was funded [4]. This initiative was further strengthened via additional collaborations with the Defense Advanced Research Projects Agency (DARPA), leading to a coordinated effort to launch the “Tissue Chips for Drug Screening” program in 2012. More recently, their application has broadened to a multitude of disciplines. Researchers have since developed microphysiological systems (MPSs), also known as multi-organ tissue chips (MOTCs), established Tissue Chip Testing Centers, to validate their efficacy for disease modeling, and have sent TCs to the International Space Station U.S. National Laboratory to study the effects of microgravity on human cells [5]. (To avoid confusion, the terms "tissue chips" and "microphysiological systems" will be used exclusively throughout the rest of this review, except in cases where the original authors used the alternate terminology.)
2. Introduction of TCs and MPs
With TCs and MPSs (Figure 1) poised to become integral components of the drug discovery and regulatory process, the working definitions of the terms "tissue chips" and "microphysiological systems" need to be established. "Tissue chips" can be defined as “engineered in vitro devices that can be used to model both structure and function of working units in the body, including organs such as the brain, heart, lungs, liver, gut, pancreas, and kidneys and tissues such as skeletal muscle, adipose tissue, and bone.” Typically, such models utilize cells (preferably of human origin, to avoid interspecies differences), extracellular matrix (ECM), and biomaterials to fabricate 3D multicellular constructs in environments where cellular interactions (cell–cell, cell–ECM), biomechanical stresses (shear, pressure, stretch), bioelectrical signals, and soluble factor signaling (certain hormones, growth factors, cytokines) are all replicated to accurately model in vivo-like responses. "Microphysiological systems" can be defined as “engineered multi-tissue/organ systems constructed using two or more tissue chips or by incorporating multiple interconnected “tissue”/”organ” chambers on one chip to recreate communications among different tissues, organs, and/or organ systems to model systemic interactions in the context of normal physiology, disease, or testing of candidate therapeutics.” Communication among “tissues” or “organs” incorporated in MPSs can be established through multiple approaches, depending upon the specific relationships of interest. For instance, aspects of the cardiovascular system can be recapitulated by employing flexible tubing (if connecting multiple TCs) or microfluidic channels (if all “tissues” or “organs” are housed on one chip), in combination with pumps, to provide for the perfusion of media among different chambers, thus allowing soluble factor signaling to take place as it would in vivo. Additional or alternative modes of communication can be enabled by incorporating aspects of the nervous, immune, endocrine, and lymphatic systems.
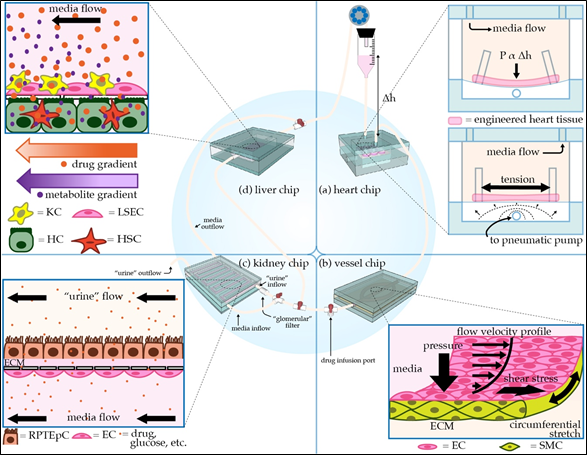
Figure 1. Schematic of modular microphysiological system. (a) Three-dimensional heart chip with cardiomyocytes (CMs) and stromal cells suspended in hydrogel between posts. The chip mimics the pressure-volume changes seen in the left ventricle. The “diastolic filling pressure,” which is directly proportional to the fluid reservoir’s height, pushes the flexible polydimethylsiloxane (PDMS) membrane downward, stretching the cardiac fiber. A pneumatic pump then generates “systolic pressure” in the lower air-filled chamber, returning the membrane and cardiac fiber to the baseline stretch. (b) Perfusable 3D vessel chip with microvessels composed of endothelial cells (ECs) and smooth muscle cells (SMCs) surrounded by an extracellular matrix (ECM), with key tunable parameters indicated. (c) Three-dimensional kidney chip mimicking the proximal tubule and adjacent peritubular capillary. To mimic the proximal tubule, renal proximal tubule epithelial cells (RPTEpCs) are cultured upon a bed of ECM. RPTEpCs have a prominent brush border, as they would in vivo. The underlying porous membrane recapitulates the selective barrier function of the tubule wall. In addition to the upstream drug infusion port, there is a valve allowing for media to bypass the kidney chip, as well as a valve splitting the kidney chip media inflow. One inflow branch passes through a “glomerular” filter and enters the proximal tubule chamber as “urine.” The remaining unfiltered media flows into the bottom vascular chamber, the superior aspect of which is lined with ECs. Other applications of this chip include modeling transport phenomena related to drugs or other key molecules. (d) Liver chip with liver sinusoidal endothelial cells (LSECs) and Kupffer cells (KCs) lining the “sinusoid,” a porous membrane mimicking the perisinusoidal space, and hepatocytes (HCs) and hepatic stellate cells (HSCs) cultured below the membrane. HCs have microvilli projecting towards the “perisinusoidal space,” as they would in vivo.
Both TCs and MPSs represent miniaturized versions of the human body and require microscale bioengineering technologies to organize cells into “tissues” and facilitate fluid flow. Therefore, microfabrication techniques and microfluidic technologies play an essential role in the construction and operation of TCs and MPSs. Each tissue type requires efficient design consideration to enable proper functionality. Design parameters include tissue architecture (cell types and organization, ECM composition), blood flow (oxygen and nutrient delivery, waste removal), and physical stresses (shear, pressure, stretch) associated with the target organ/tissue. This review focuses on providing a basic understanding of the tissue-level organization and function of four critical organs that are involved in metabolizing or excreting drugs (liver and kidney) or are frequently damaged by off-target drug toxicity (heart and blood vessels). Essential design considerations in the development of TC models of each organ/tissue are examined, and current efforts and past successes in TC models’ development are summarized. While this review focuses on the heart, vasculature, liver, and kidneys, more information on other organ systems can be found in the following excellent articles focused on the brain [6], lungs [7], GI system [8], pancreas [9], and reproductive system [10]. Additionally, the following review articles also provide comprehensive overviews of TCs and their use in drug testing [11,12] and disease modeling [13].
This entry is adapted from the peer-reviewed paper 10.3390/mi12020139