Interleukin (IL)-4 and -13 are structurally and functionally related cytokines sharing common receptor subunits. They regulate immune responses and, moreover, are involved in the pathogenesis of a variety of human neoplasms. In this entry, their possible roles in gastric cancer were shown as an example.
- interleukin-4
- interleukin-13
- interleukin-4 receptor
- interleukin-13 receptor
1. Introduction
Gastric cancer (GC) and colon and rectal cancer (CRC) are common malignancies of the digestive system [1,2]. Despite advances in earlier detection, multimodal treatment, and surgical management, the prognosis of both entities is still unsatisfactory [3]. CRC is the second leading cause of all tumor deaths in the United States [3], and stomach cancer is the third leading death cause of cancer-related deaths worldwide [4]. Alternative or additional treatment strategies especially for advanced tumor stages are desperately needed to overcome drug resistance, enhance chemosensitivity, inhibit tumor cell proliferation, and induce apoptosis in order to further improve outcome [1,2,5,6,7,8,9].
More and more evidence has been provided in recent years that interleukin-4 (IL-4), interleukin-13 (IL-13), and their receptors play an important role in cancer cell proliferation and other biological behaviors, such as migration and invasion enhancing the malignant phenotype [10,11,12]. Moreover, IL-4/IL-13 and their receptors have been also associated with apoptosis, chemosensitivity, and prognosis in various cancers [13,14,15]. IL-4 and IL-13 are also involved in the crosstalk with the tumor microenvironment (TME) by activating tumor-associated macrophages and myeloid-derived suppressor cells, which have tumor promoting functions [16,17]. Immune surveillance against established metastatic mammary cancer is negatively regulated by IL-13 in mice [18].
2. Summary of the IL-4/-13 Signaling Pathway
IL-4, first described in 1981, is a secreted cytokine that, in its physiologic function, can regulate antibody production, hematopoiesis, and inflammation, and is also involved in the development of effector T-cell responses [19]. The closely related IL-13, first described in 1993, is a human lymphokine that can regulate inflammatory and immune responses [20]. IL-4 and IL-13 are essential for the induction and persistence of the type 2 immune response, and they are associated with multiple atopic diseases, such as asthma and atopic dermatitis [21]. IL-4 and IL-13 are mainly produced by immune cells, such as CD4-T-cells, basophils, eosinophils, and natural killer T (NKT) cells [22].
The structure of IL-4 receptor (IL-4R), IL-13 receptor (IL-13R), and the positions of the intracellular signaling molecules of them have been summarized in several articles [23,24,25,26]. There are three different kinds of IL-4 receptors (Figure 1). IL-4 binds to the IL-4Rα chain, then recruits the IL-2Rγ-common (γc) chain (type I IL-4R) or the IL-13Rα1 chain (type II IL-4R) to form a receptor complex that can initiate signal transduction [25]. The type III IL-4R is formed by all the three chains [27]. IL-13 can also signal via three different receptors (Figure 1). The type II IL-13R complex has the same components as the type II IL-4R [28]. IL-13R type I (IL-4Rα/IL-13Rα1/IL-13Rα2) and type II (IL-4Rα/IL-13Rα1) are expressed in non-hematopoietic cells, while type III (IL-4Rα/IL-13Rα1/γc) is only expressed on the surface of hemocytes [27]. Overall, this results in a possible complex web of IL-4– and IL-13–mediated signaling pathways [29].
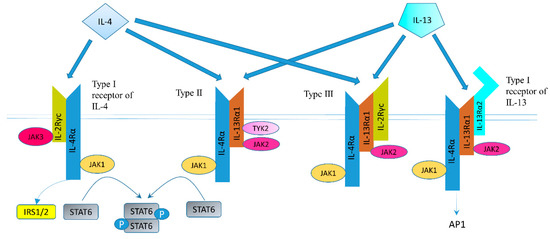
Figure 1. Receptor types and signal transduction of IL-4R and IL-13R. There are three different kinds of IL-4 receptors. IL-4 binds to the IL-4Rα chain, then recruits the IL-2Rγ-common (γc) chain (type I IL-4R) or the IL-13Rα1 chain (type II IL-4R) to form a receptor complex that can initiate signal transduction. The type III IL-4R consists of all three chains. IL-13 also have three different receptors. IL-13 receptor type I (IL-4Rα/IL-13Rα1/IL-13Rα2) and type II (IL-4Rα/IL-13Rα1) are expressed in solid tumors, while type III (IL-4Rα/IL-13Rα1/γc) is expressed in hemocytes only. IL-13 binds IL-13Rα1 with a low affinity and complexes with the IL-4Rα (type II receptor). IL-13 binds to the IL-13Rα2 with a high affinity. IL-13 can bind to a soluble IL-13Rα2 receptor, which has no downstream signaling, or bind to transmembrane IL-13Rα2 and activate AP-1. Figure sketch adapted from reference [27].
With regard to the IL-13Rα2 chain, IL-13 is not the sole ligand. For example, chitinase-3-like protein 1 (CHI3L1) could bind to IL-13Rα2 and regulate oxidant injury, apoptosis, and melanoma metastasis [30]. Transmembrane protein 219 and CD44 play an important role in IL-13Rα2 mediated signaling which is induced by CHI3L1 [31,32].
Altogether, IL-4R and IL-13R share two receptor chains (IL-4Rα and IL-13Rα1) and can mediate common, but also diverse biological functions [27]. Both IL-4 and IL-13 phosphorylate and activate signal transducer and activator of transcription (STAT) 6 [27]. STAT3, STAT5, and STAT1 can also be activated, but to a lesser degree [23]. IL-4 can signal through IRS-2 (generally expressed by hematopoietic cells) or IRS-1 (generally non-hematopoietically expressed) [23]. As mentioned above, IL-13 could bind to the IL-13Rα2 chain, which has a very high affinity for IL-13. The downstream signaling involves AP-1 family members c-jun and Fra-2 [33]. IL-13Rα2 can inhibit downstream signals of IL-13R and IL-4R through regulating STAT6 [34,35].
3. IL-13/IL-13R in Gastric Cancer
IL-13Rs are overexpressed in several human solid cancer cell lines [36,37]. Our group demonstrated that IL-13R and IL-4R were expressed in pancreatic cancer cell lines, such as PANC-1, MIAPaCa-2, and CAPAN-1 [38]. Their proliferation was inhibited by Pseudomonas exotoxin (PE) combined to IL-13 or IL-4, demonstrating the receptor’s functionality [38]. IL-13Rα2 is expressed in HS766T and MIAPaCa-2 pancreatic cancer cells, as well [36]. One recombinant chimeric protein IL-13PE was found highly cytotoxic to GC cell line CRL1739, which also expressed the type II IL-4R receptor (Figure 1) binding both IL-4 and IL-13 [39]. IL-13Rα2 is also expressed in GC cell lines MKN-45, AGS and MGC308 [32,40].
Gabitass et al. evaluated plasma IL-13 and IL-4 levels in 131 patients (46 pancreatic cancer, 25 GC, and 60 esophageal cancer) and 54 healthy controls [41]. IL-13 levels in patients’ plasma were significantly higher in all the three cancer patients compared with controls [41]. In another study, Lin et al. evaluated IL-13Rα2 expression in tissue microarrays of 507 GC patients [15]. They found the overexpression of the IL-13Rα2 chain in cancer tissue was associated with poor prognosis after gastrectomy [15].
Chen and coworkers showed that CHI3L1 secreted by M2 macrophage could promote the metastasis of GC cell lines MKN-45 and AGS by binding to the IL-13Rα2 chain [40]. The mechanism is mediated by activating the mitogen-activated protein kinase signaling pathway, which upregulates the matrix metalloproteinase genes [40]. Geng et al. found CD44v3 could bind to both CHI3L1 and IL-13Rα2 in GC cell lines AGS and MGC308 [32]. In this study, CHI3L1 expression was positively related to GC invasion depth and lymph node status in 100 GC tissues from patients [32].
4. IL-4/IL-4R in Gastric Cancer
Human GC cell lines such as CRL1739 express IL-4R [42]. IL-4 inhibited proliferation of HTB-135 GC cells by down-regulating G0-G1 cell cycle nuclear-regulating factors, including retinoblastoma gene product, c-myc, and cyclin D1 [43]. IL-4 could cause G1 phase arrest in the GC cell line CRL 1739 by binding to IL-4Rα and γc (type I IL-4R) [42]. IL-4 could also inhibit the growth of GC cells and this effect was positively related with IL-4R expression level of the respective cell lines [44]. The expression was detected by flow cytometry using biotin-labeled IL-4. It remains unclear, however, what type of IL-4R causing inhibition of GC cell growth was expressed (Figure 1).
Gabitass et al. found plasma IL-4 levels in 25 GC patients were significantly higher than in 54 healthy controls [41]. Cárdenas et al. found serum IL-4 levels in 17 GC patients were significantly elevated comparing with 30 healthy individuals by sandwich ELISA [45]. In their study, elevated serum levels of IL-4 indicated a higher risk of mortality, but there is no statistical association [45]. Orea and co-workers studied a total of 30 biopsies of GC by immunohistochemistry [46]. They found a significantly higher expression of IL-4 in stages I and II than in stages III and IV, pointing to a possible growth inhibitory effect of IL-4 in GC [46].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020727
