It is widely recognized that many chronic infections of the human body have a polymicrobial etiology. These include diabetic foot ulcer infections, lung infections in cystic fibrosis patients, periodontitis, otitis, urinary tract infections and even a proportion of systemic infections. Treatment of mixed infections poses serious challenges in the clinic as a plethora of interactions establish among community members that may greatly affect the expression of virulence factors and susceptibility to antimicrobials of individual species in the community. Therefore, new strategies able to target multiple pathogens in mixed populations need to be urgently developed and evaluated. In this regard, antimicrobial or host defense peptides (AMPs) deserve particular attention as they are endowed of many favorable features that may serve to this scope. An updated overview of studies addressing the therapeutic potential of AMPs in mixed infections is provided, highlighting the opportunities offered by this class of antimicrobials in the fight against polymicrobial infections, but also the limits that may arise in their use for this type of application.
- antimicrobial peptides
- host defense peptides
- polymicrobial infections
- biofilm infections
1. Introduction
A major player in antibiotic tolerance and virulence during polymicrobial infections is biofilm formation [33]. Within a biofilm, an abundant extracellular polymeric substance (EPS) protects all microbial cells (including the non-producers) from a variety of harmful stimuli, including antibiotics and host defense factors (Figure 1). Adam et al. reported a striking example of how EPS alters antibiotic susceptibility in mixed infections [34]. They demonstrated that while an EPS-nonproducing mutant strain of S. epidermidis is normally highly sensitive to vancomycin, it is protected from the same antibiotic when grown in co-cultures with C. albicans [34]. On the other hand, the abundant EPS produced by the wild-type strain of S. epidermidis (RP62A) can inhibit fluconazole penetration in mixed fungi-bacteria biofilms, protecting C. albicans from the action of the antifungal drug [34]. Recently, an interesting mechanism by which C. albicans may promote multidrug tolerance in S. aureus was proposed [35]. S. aureus grown in dual cultures with C. albicans was found to display decreased intracellular ATP levels and lower membrane potential as compared to cultures lacking C. albicans. C. albicans-mediated nutrient deprivation was shown to cause decreased metabolic activity in S. aureus, inducing the formation of persisters, dormant cells highly tolerant to antibiotic treatment. Members of a polymicrobial biofilm may also produce antibiotic-modifying enzymes (e.g., β-lactamases) of which not only the producing species, but also the co-infecting species may benefit. Interspecies horizontal gene transfer is another mechanism that might facilitate the acquisition of antibiotic-resistant genes within a polymicrobial biofilm [33].
Despite the numerous examples correlating interspecies interactions in mixed infections with variations in pathogenicity and the antibiotic susceptibility of individual organisms, antibiotic therapies are often directed towards the most relevant pathogen, disregarding the consequences that the presence of other bacterial species may have in the pathogenicity and the response to antimicrobial therapy [36]. Therefore, new strategies targeting multiple pathogens in mixed populations and considering the multifaceted interactions that are established in the community need to be evaluated.
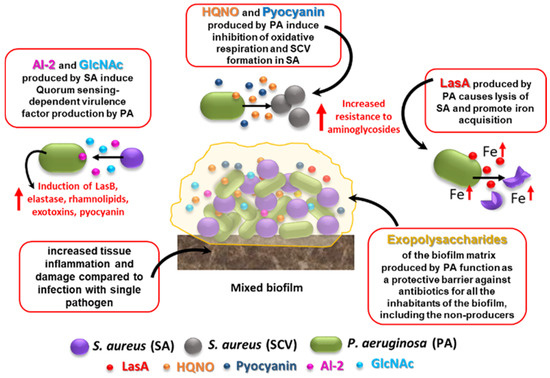
Figure 1. Effects of S. aureus–P. aeruginosa mixed infection on resistance and virulence as compared to single-species biofilms. Some of the best characterized interactions that occur between the two species in mixed biofilms are shown. See text for details. HQNO: 4-Hydroxy-2-Heptylquinoline N-Oxide; SCV: small colony variant; AI-2: autoinducer 2; GlcNAc: N-acetyl glucosamine, a component of bacterial peptidoglycan.
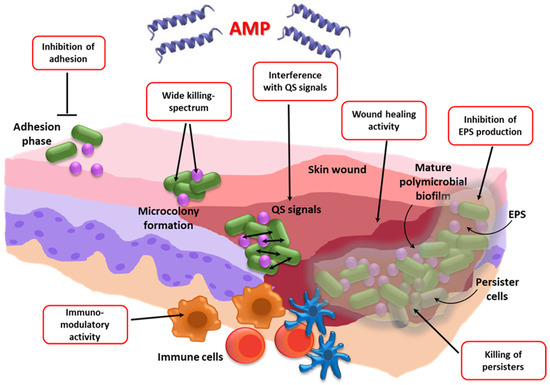
The interest in the use of antimicrobial or host defense peptides (AMPs) as antibiofilm agents has rapidly grown over the last few decades [37]. Many AMPs have shown activity in killing cells in biofilms, interfering with EPS production and stability, inhibiting QS-dependent biofilm formation, or preventing microbial adhesion when used to coat medical implants [37,38]. A manually curated database of AMPs specifically assayed against microbial biofilms (http://www.baamps.it/) was issued for the first time in 2015 [39] and has stimulated the development of several computational approaches to accurately predict anti-biofilm peptides [40,41,42]. Such approaches have revealed a prevalence of positively charged and aromatic residues, and the selective presence of some dipeptides and sequence motifs in biofilm inhibiting peptides (BIP) as compared to non-BIP, aiding the choice of potential AMP-candidates to direct toward preclinical development.
Despite the keen interest in AMPs as antibiofilm agents, their possible use against biofilm-associated polymicrobial infections is a relatively poorly investigated research area, but it has the potential to offer innovative and effective solutions for the treatment of co-infections (Figure 2).
2. Bacteria–Bacteria Mixed Infections
2.1. Wound Infections
AMPs are part of the innate skin defense mechanisms providing a first-line barrier to microbial insult [73]. Skin AMPs include β-defensins (BD), cathelicidins (human hCAP18/LL37), RNase 7, chemerin, and secretory leukocyte protease inhibitor (SLPI) [73]. For example, hCAP18/LL37, one of the best-characterized peptides in skin defense, is upregulated in the epidermis as a result of skin injury and infection, while mice deficient in the murine homolog of hCAP18/LL37 (CRAMP) are more susceptible to serious cutaneous streptococcal infections [74], highlighting the importance of AMPs in skin protection against bacteria.
AMPs hold promise as new therapeutic agents for infected wounds due to their broad activity spectrum, antibiofilm potential, immunomodulatory action, angiogenetic and wound-healing properties, and their ability to stimulate cell proliferation and migration [45,75,76,77]. However, only a relatively small number of AMPs have been tested as a new therapeutic strategy to prevent or treat polymicrobially infected wounds (Table 1). For instance, Chung and coworkers designed and synthetized a new short AMP (named DRGN-1), a derivative of the VK25 peptide found in the plasma of the Komodo dragon (Varanus komodoensis), a large species of lizard found on the Indonesian island of Komodo [49]. They demonstrated that the peptide significantly inhibits single species and mixed-species biofilms of P. aeruginosa and S. aureus in vitro at 24 h, as evaluated by the crystal violet staining of biofilms and confocal microscopy. The peptide was also tested in a mouse wound infection model. To this aim, full-thickness, 6 mm diameter round wounds were overlaid with a mixed biofilm of P. aeruginosa and S. aureus grown on agar for two days, and the kinetics of wound closure and the bacterial load were evaluated after peptide treatment. The results obtained demonstrated the ability of DRGN-1 to accelerate wound closure and reduce the bacterial count of both species. The efficacy of DRGN-1 to stimulate keratinocyte migration in a scratch-wound closure assay was also demonstrated, further stressing the potentiality of the peptide in the therapy of infected wounds.
The antimicrobial efficacy of individual AMPs can be greatly enhanced by combining them with other AMPs or with other antimicrobial agents [78]. Combination therapies have the undisputed advantage of being able to reduce the insurgence of resistance as well as the active concentrations of the combined drugs, with consequent attenuation of cytotoxicity and possible side effects. Gomes et al. recently assessed the combination of two AMPs, pexiganan and nisin, for their ability to control polymicrobial diabetic foot infections [50]. When tested against planktonic and biofilm cells of S. aureus, the dual AMP displayed an increased activity compared to pexiganan used alone, but this was not the case for P. aeruginosa monocultures or dual species cultures. It was suggested that the scarce effect elicited by adding nisine to pexiganan to target P. aeruginosa was due to nisin’s mode of action, which relies on its ability to bind lipid II with the consequent inhibition of cell wall biosynthesis [79]. As lipid II is located in the cytoplasmic membrane, the presence of an outer membrane in Gram-negative bacteria may hamper the peptide’s ability to reach its target, with reduced antimicrobial efficacy. A DFU collagen three-dimensional (3D) model was used to evaluate further the efficacy of the locally delivered dual AMP, incorporated in a guar gum biogel. When S. aureus and P. aeruginosa were inoculated as a dual species inoculum into the model, the strong antibacterial activity of the dual AMP biogel was observed against S. aureus, resulting in bacterial eradication from three different areas of the collagen scaffold. In contrast, the activity of the dual AMP biogel was null or scarce against P. aeruginosa, which was detected, instead, in all the areas of the model. These results highlight that P. aeruginosa might be a bacterial species particularly difficult to target with both conventional antibiotics and AMPs. In addition, the data obtained suggest that the mechanisms of action of the peptide(s) employed should be taken into consideration to target all the species of a mixed community with equal efficiency. Jorge and coworkers explored another AMP-based combination strategy, testing colistin sulfate salt (CST) combined with the AMPs temporin A (TEMP-A), citropin 1.1 (CIT-1.1) or tachyplesin I linear analogue (TP-I-L) against single and dual species biofilms of the two major wound pathogens P. aeruginosa and S. aureus [51]. They demonstrated synergistic/additive or indifferent activity against 24-h-old dual species biofilms, depending on the antimicrobial combination and strain tested (reference strains, or MDR clinical isolates). Although in mixed biofilms the initial bacterial number was the same for the two species, at 24 h the biofilms were predominantly composed of P. aeruginosa, suggesting the establishment of competitive interactions between the two species during the incubation period. The AMP concentration required to target the dual species biofilms was overall higher than that required to treat mono-species biofilms, with some of the combinations demonstrating a high level of cytotoxicity against mammalian cells [51].
2.2. Respiratory Infections
Host AMPs represent key elements in the innate defense of the lung, with defensins and cathelicidins being the peptide families most represented in the airway secretions [83]. They can contribute to host defense in the lung by killing the pathogens as well as by modulating the host inflammatory response. These favorable properties have stimulated investigations on their exogenous administration to prevent/treat infections [84,85,86]. Only a few AMPs have been tested in lung polymicrobial infections. One of these peptides is the Tachyplesin III, a β-sheet peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus), which has been tested in bacterial co-infection pneumonia [55]. As compared to mono-bacterial infection, the intranasal co-infection of mice with MDR P. aeruginosa and A. baumannii caused a more serious disease, with increased pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines (MCP-1/MIP-2) and reduced survival. The pretreatment of mice with a single dose of Tachyplesin III (10 mg/kg, i.v.) could prolong mice survival and significantly reduce the total bacterial count in the bronchoalveolar lavage fluid, as compared to the untreated or meropenem-treated control mice groups. Interestingly, the peptide was also found to reduce the serum level of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and to decrease inflammatory cell infiltration, vascular leakage, and alveolar disruption in the Tachyplesin III-pretreated group as compared to the co-infected group or the meropenem-treated group. Finally, when tested in vitro, the peptide displayed the ability to enhance the phagocytic function of mouse alveolar macrophages, suggesting that its prophylactic efficacy might be due to multimodal mechanisms of action.
2.3. Oral Infections
Several AMPs are naturally produced in the oral cavity as part of the innate immune system, and they are believed to greatly contribute to maintaining microbial homeostasis and health status in the oral district [90,91]. As many AMPs have shown good activity against oral bacteria, their use to prevent/treat oral infections seems promising, although their antimicrobial potency in the oral cavity might be challenged by the presence of saliva or crevicular fluid, due to high salt concentration, the presence of proteases of host/bacterial origin, or sequestration by the macromolecules present in such fluids [92,93,94]. Wang and coworkers reported the ability of a synthetic cationic AMP, Nal-P-113, to exert a significant bactericidal activity against oral pathogens, i.e., Streptococcus gordonii, Fusobacterium nucleatum and P. gingivalis, in both planktonic and polymicrobial biofilm states [56]. The peptide is the optimized derivative of another peptide, P-113 (AKRHHGYKRKFH-NH2), in which histidine residues were replaced with the bulky amino acid β-naphthylalanine, resulting in increased salt resistance [95]. Nal-P-113 retained more than 85% integrity after 8 h incubation in phosphate buffered saline (PBS), saliva from healthy donors, brain heart infusion medium, and bovine calf serum. Importantly, at a concentration that only causes slight damage to normal oral cells (1.28 mg/mL), Nal-P-113 was able to eradicate triple strain biofilms of S. gordoni, F. nucleatum and P. gingivalis, while the minimum biofilm eradication concentrations of penicillin and metronidazole were 2 mg/mL and 80 mg/mL, respectively. It is noteworthy that many AMPs (e.g., beta-defensins, human neutrophil defensins, the human cathelicidin LL-37) have shown lipopolysaccharide (LPS)-neutralizing activities against periodontopathogens, causing the inhibition of the IL-1β, IL-8, and intercellular adhesion molecule 1 (ICAM-1) expression induced by LPS from P. intermedia and T. forsythia in THP-1 cells and human gingival fibroblasts [96]. Altogether, these results suggest that AMPs may be considered as preventive and therapeutic agents against mixed bacterial infections, such as periodontitis, by killing the pathogens as well as by reducing the activity of LPS and disease-associated inflammation.
2.4. Sepsis
2.5. Infections of the Lower Female Reproductive Tract
Zhu and coworkers tested the therapeutic potential of the AMP HPRP-A2 in combination with chlorhexidine acetate (CHA) in a rat vaginitis infection model [60]. They infected the animals intravaginally with a 1:1 suspension of E. coli and S. aureus. After 8 days of treatment with HPRP-A2, CHA or their combination, the vaginal bacterial count was evaluated. In both low-dose and high-dose treatment groups a statistically significant reduction in the CFU counts of both bacterial species was observed as compared to the control animals. The highest rate of inhibition was observed in the animals treated with the HPRP-A2-CHA combination. For instance, as compared with the untreated controls, the reduction in the CFU count of E. coli and S. aureus treated with a high dose of HPRP-A2 or CHA alone ranged from 56.9 to 67.3%, while their combination reached an inhibition of 99.9%, stressing the possibility of successfully combining AMPs with conventional drugs to obtain a synergistic therapeutic effect.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020482
