Grifola frondosa (G. frondosa) is a Basidiomycetes fungus that belongs to the family of Grifolaceae and the order of Polyporales.
- Grifola frondosa
- maitake mushroom
- polysaccharide
1. Introduction
In Japan Grifola frondosa (G. frondosa) edible fruiting body is known as maitake. In Japanese, mai means dance and take means mushroom. G. frondosa is known as “hui-shu-hua” (grey tree flower) in Chinese, possibly due to its appearance. G. frondosa grows around the stumps of broadleaf trees or trunks and is edible when young. The environment of the northeastern part of Japan is suitable for the growth of G. frondosa. The temperate forests in eastern North America, Europe and Asia are also ideal for its growth. Meanwhile, it is a common mushroom in the Unites States and Canada, known as sheep’s head, king of mushrooms, hen-of-the-woods, and cloud mushroom [1].
Japan was one of the countries that first started the artificial cultivation of G. frondosa in the mid-1980s. There are in general three methods for the artificial cultivation of the G. frondosa fruiting body, they are bottle culture, bag culture and outdoor bed culture. Bag culture is the most popular cultivation method in Japan [2] because of its advantages such as the low cost of plastic bags, small space requirements and easily-controlled indoor environment. Bag culture can achieve higher yields of mature G. frondosa mushrooms than bottle culture and requires a shorter cultivation time than outdoor bed cultures. As shown in Figure 1 [2], the major steps of bag cultivation include substrate preparation, substrate sterilization, mycelium inoculation and incubation. In addition to the fruiting body, there is also an increasing demand for G. frondosa’s mycelium and its bioactive metabolites. Solid-state fermentation (SSF) [3] and submerged fermentation [4] are two common methods of mycelium cultivation. A common substrate for SSF is sawdust supplemented with rice bran or wheat bran [5]. Submerged or liquid fermentation is usually more efficient, providing a higher mycelial productivity in a shorter time, requiring smaller plant space and allowing for more effective product quality control [6]. A typical submerged fermentation process is presented in Figure 2.
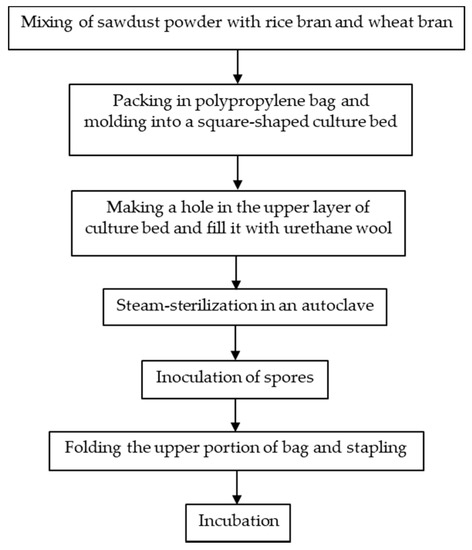
Figure 1. A typical bag culture procedure for the G. frondosa fruiting body.
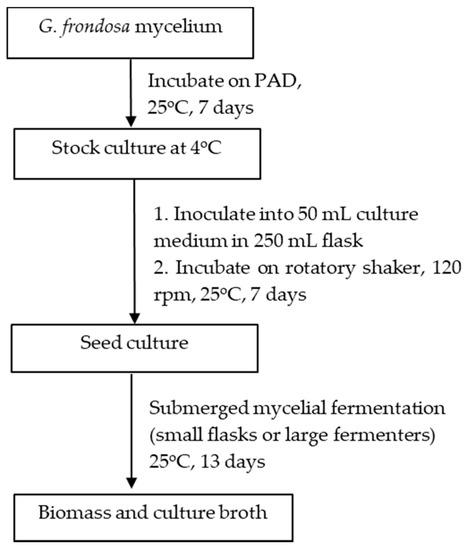
Figure 2. Submerged culture fermentation of the G. frondosa mycelium adapted from [4].
G. frondosa is edible and is regarded as a healthy food because it is a good source of protein, carbohydrates, dietary fiber [7][8][9][10][11][12][13], vitamin D2 (ergocalciferol) [13][14][15] and minerals (K, P, Na, Ca, Mg) [7][9][12][15][16], with low fat content and caloric value [15]. G. frondosa is delicious, with a sweet and umami taste, which is mainly attributed to its high trehalose, glutamic and aspartic amino acid and 5′-nucleotide content [10][11][13][17]. Due to its delicious and special taste, G. frondosa is not only used as a food ingredient, but also as a food-flavoring substance in dried powder form. Apart from its high nutraceutical value, G. fondosa is reported to possess a wide range of pharmacological effects. G. frondosa was first discovered to have antitumor activity in the 1980s from hot water extracts of the G. frondosa fruiting body [16][17]. The major bioactive components were found to be β-glucans [17][18][19][20]. The D-fraction, a β-glucan complex with about 30% protein, was first discovered by Nanba’s group in the late 1980s [21]. Since then, the D-fraction has been widely studied and gradually developed into commercially available complementary medicines and healthcare products. In addition to the D-fraction, there are many other bioactive polysaccharide fractions that are obtained from G. frondosa, such as the MD-fraction [22], X-fraction [23], Grifolan [24], MZ-fraction [25] and MT-α-glucan [26]. The different polysaccharide fractions isolated from G. frondosa possess various bioactive effects such as immunomodulation [24], antitumor [25], antivirus [27], antidiabetic [26] and anti-inflammation [28]. In recent years, an increasing number of studies have attributed or linked the health and therapeutic effects of G. frondosa polysaccharides to their capacity for modifying gut microbiota, microorganisms that play an important role in human health and diseases. In particular, gut microbiota play a role in maintaining immune homeostasis, which may have a connection to the antitumor effects of polysaccharides [29]. The regulation of gut microbiota composition by G. frondosa polysaccharides has also been suggested to contribute to the treatment of metabolic disorders such as non-alcoholic fatty liver disease (NAFLD) [30] and diabetes [31], indicating their potential for preventing or treating hyperglycemia and hyperlipidemia. Apart from polysaccharides, other molecular fractions isolated from G. frondosa fruiting bodies or mycelial biomass have shown promising medicinal values as well. For instance, the protein components of G. frondosa, including glycoprotein, have shown anti-tumor [32], immune-enhancing [33], anti-diabetic, anti-hypertensive, anti-hyperlipidemic [34] and anti-viral effects [35]. Moreover, other small biomolecules in G. frondosa have been found to possess health benefits such as anti-inflammation [36], hypoglycemia [37], antitumor [38] and antioxidation [39].
2. Chemical and Nutritional Compositions
2.1. Proximate Composition
Generally, proximate composition is determined by the methods suggested by the Association of Official Analytical Chemists (AOAC). The total carbohydrate content can be calculated by subtracting the percentages of ash, crude fat and protein [7][40]. For the determination of crude protein, the nitrogen conversion factor is 4.38 instead of the usual 6.25, due to the large amount of chitin that is usually contained within the fungus, a component that may interfere with the correct calculation of the result of total nitrogen [41].
As shown in Table 1, G. frondosa is made up of around 83–96% moisture and 4–17% dry matter in its fresh fruiting body [7][8][9][10][11][12][13] and mycelium [11][17][42], indicating the watery texture of G. frondosa. Carbohydrates and protein are the major constituents contributing to the dry weight of G. frondosa, taking up around 70–80% and 13–21%, respectively, of the fruiting body. Based on the average values of component percentage, it could be found that the mycelium of G. frondosa has a similar moisture content, a lower content of carbohydrate and crude ash and a higher content of crude fat and protein, compared with the fruiting body of G. frondosa.
Table 1. Proximate composition of G. frondosa’s fruiting body and mycelium.
| Components 1 (%) |
Fruiting Body | Mycelium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] * | [9] # | [12] # | [10] 2,# | [13] # | [11] 2,# | [7] 2,# | Average | [43] | [44] 2 | [11] 2 | Average | |
| Moisture | 83.1 | 89.1 | 90.9 | 86.1 | 90.4 | 95.6 | 95.2 | 90.1 ± 4.5 | 84.8 | 96.7 | 92.3 | 91.3 ± 6.0 |
| Dry matter 3 | 16.9 | 10.9 | 9.1 | 13.9 | 9.6 | 4.4 | 4.8 | 9.9 ± 4.5 | 15.2 | 3.3 | 7.7 | 8.7 ± 6.0 |
| Carbohydrate 4 | 70.4 | 74.9 | 72.3 | 68.8 | 71.8 | 66.3 | 70.3 | 70.7 ± 2.7 | 66.3 | 45.0 | 60.4 | 57.2 ± 11.0 |
| Crude ash | 6.5 | 4.8 | 6.6 | 7.0 | 7.1 | 6.2 | 4.9 | 6.1 ± 0.9 | 6.4 | 4.0 | 4.7 | 5.0 ± 1.3 |
| Crude fat | 4.5 | 1.5 | 3.3 | 3.1 | 2.4 | 6.5 | 5.6 | 3.8 ± 1.8 | 4.2 | 24.7 | 6.5 | 11.8 ± 11.2 |
| Crude protein | 18.6 | 18.9 | 17.8 | 21.1 | 18.8 | 21.0 | 19.2 | 19.3 ± 1.3 | 23.1 | 26.4 | 28.4 | 26.0 ± 2.7 |
1 Moisture and dry matter were based on fresh weight; others were presented based on dry weight. 2 For easy comparison, only the mean value is used. 3 The dry matter was represented by (1 − moisture/total) × 100%. 4 Amount of carbohydrate was calculated by subtracting crude ash, crude fat and crude protein. * Fruiting body was grown naturally. # Fruiting body was grown artificially.
According to the research findings of Kurasawa and coworkers [8], the composition of the G. frondosa fruiting body resembles that of normal cultivated mushrooms. It is worth mentioning that the crude fat content of the G. frondosa fruiting body is generally lower than the average crude fat content in cultivated mushrooms (4.3%), and the amounts of protein and carbohydrates are slightly higher than the average of other mushrooms (17.2% and 70.3%), indicating the excellent nutritional values of G. frondosa.
2.2. Soluble Sugar Content
The content of soluble sugar within G. frondosa is mostly determined by the method described in the research work of Ajlouni and coworkers [42]. As shown in Table 2, the total sugar content in G. frondosa is higher in the fruiting body (90–190 mg/g) than in the mycelium (70–90 mg/g). The content of total sugar in the fruiting body of G. frondosa is also superior to some edible mushrooms such as Lactarius glaucescens and Craterellus odoratus [45], which may be one of the reasons for the good taste of G. frondosa. Table 2 also shows variations in both total soluble sugar content and individual sugar content among different G. frondosa samples, which may be attributed to factors such as cultivation period and cultivation environment. Trehalose, a disaccharide that comprises two molecules of glucose, is the major sugar component of both the fruiting body and mycelia of G. frondosa [9][10][11][43][44]. Compared with the amount in the mycelium (40–60 mg/g), the fruiting body contains more trehalose than the mycelium, around 50–160 mg/g in dry weight. In addition to trehalose, the fruiting body also contains glucose and mannitol, whereas the mycelium has glucose and mannitol, together with arabitol and fructose.
Table 2. Soluble sugar content of G. frondosa fruiting body and mycelium in dry weight.
| Component | Fruiting Body (mg/g Dry wt.) |
Mycelium (mg/g Dry wt.) |
||||
|---|---|---|---|---|---|---|
| [9] # | [10] 1,# | [11] 1,# | [43] | [44] 1 | [11] 1 | |
| Arabinose | n.d. 2 | n.d. | n.d. | n.d. | n.d. | 5.37 |
| Arabitol | n.d. | n.d. | n.d. | n.d. | 12.65 | 2.01 |
| Fructose | n.d. | n.d. | n.d. | 1.00 | n.d. | 2.99 |
| Glucose | 59.30 | 14.02 | 2.42 | 8.00 | 19.72 | 2.18 |
| Lactose | n.d. | n.d. | n.d. | n.d. | n.d. | 0.93 |
| Mannitol | 7.20 | 9.36 | 1.00 | n.d. | 9.92 | 2.30 |
| Mannose | n.d. | n.d. | n.d. | n.d. | n.d. | 1.92 |
| Ribose | n.d. | n.d. | 8.34 | n.d. | n.d. | 4.04 |
| Trehalose | 45.80 | 161.83 | 99.94 | 65.00 | 41.60 | 65.32 |
| Total | 112.30 | 185.21 | 111.7 | 74.00 | 83.89 | 87.06 |
1 For easy comparison, only the mean value is used. 2 Not determined or not detected. # Fruiting body was grown artificially.
2.3. Free Amino Acid Content
The content of free amino acids in G. frondosa was quantitatively measured by the method described in the research work of Mau and coworkers using HPLC [10][44]. Results regarding the free amino acid content of G. frondosa are exhibited in Table 3. The total free amino acid content in the fruiting body of G. frondosa is around 15–60 mg/g in dry weight, which is higher than that in many other edible mushrooms, such as Dictyophora indusiata and Tricholoma giganteum [10]. The mycelium of G. frondosa contains a relatively higher total free amino acid content in comparison with the fruiting body. There is also a great variety of amino acids in G. frondosa. There are around eighteen kinds of free amino acids, including essential amino acids such as L-histidine and L-methionine, in both the fruiting body and the mycelium of G. frondosa, indicating that G. frondosa is an excellent source of amino acids.
Table 3. Free amino acid assay of G. frondosa’s fruiting body and mycelium in dry weight.
| Component (mg/g Dry wt.) |
Fruiting Body | Mycelium | ||||
|---|---|---|---|---|---|---|
| [13] 1,# | [11] 1,# | [10] 1,# | [44] | [11] 1 | ||
| In Sawdust | In Log | |||||
| L-Alanine | 2.15 | 3.13 | 5.22 | 2.77 | 3.26 | 14.59 |
| L-Arginine | 3.02 | 3.21 | 1.66 | 0.64 | 0.97 | 12.39 |
| L-Aspartic acid | 1.61 | 1.25 | 1.88 | 0.42 | 2.75 | 19.40 |
| L-Glutamic acid | 8.01 | 9.10 | 12.62 | 0.67 | 3.76 | 2.10 |
| GABA | n.d. 2 | n.d. | 0.28 | n.d. | n.d. | 17.09 |
| Glycine | 1.53 | 1.53 | 2.46 | 0.57 | 1.93 | 7.81 |
| L-Histidine 3 | 1.53 | 0.94 | 19.50 | 0.59 | 4.10 | n.d. |
| L-Isoleucine 3 | 0.12 | 0.12 | 0.56 | 0.33 | 2.80 | 6.67 |
| L-Leucine 3 | 0.05 | 0.09 | 0.27 | 0.35 | 4.92 | 6.39 |
| L-Lysine 3 | 1.56 | 1.28 | 5.70 | 1.11 | 0.22 | 23.49 |
| L-Methionine 3 | n.d. | n.d. | 4.50 | 1.40 | 0.67 | n.d. |
| L-Phenylalanine 3 | 0.26 | 0.28 | 2.71 | 0.80 | 1.66 | 9.98 |
| L-Serine | 2.91 | 2.82 | 2.01 | 0.97 | 2.73 | 10.74 |
| L-Threonine 3 | 1.43 | 1.44 | n.d. | 4.40 | 8.23 | 10.85 |
| L-Tryptophan 3 | n.d. | n.d. | n.d. | 0.27 | n.d. | 12.01 |
| L-Tyrosine | 1.77 | 0.73 | 1.53 | n.d. | 2.15 | 17.99 |
| L-Valine 3 | 0.96 | 0.91 | 0.39 | 0.60 | 4.13 | 9.41 |
| Total | 29.26 | 29.38 | 61.29 | 15.9 | 44.28 | 180.91 |
1 For easy comparison, only the mean value is presented. 2 Not determined or not detected. 3 Essential amino acid. # Fruiting body was grown artificially.
However, large variations exist in the amount of each amino acid in G. frondosa. For instance, Mau et al. (2001) and Tsai et al. (2006) found that threonine was the major free amino acid in G. frondosa [10][44], whereas Huang and coworkers found that histidine and glutamic acid were the major free amino acids in the fruiting body (>12 mg/g) and lysine, aspartic acid and tyrosine were the major free amino acids in the mycelium (>17 mg/g) [11]. Huang and coworkers also obtained a larger amount of free amino acid from G. frondosa than other groups in both the fruiting body and the mycelium, a result that might be due to the different sources of the fruiting body and the preparation methods of the mycelium by the different research groups. The choice in cultivation substrate was also found to affect the variety and amount of amino acids in G. frondosa. As shown in Table 3, G. frondosa fruiting bodies cultivated in sawdust and log substrates have different amounts of each amino acid [13], although the total amino acid content was similar. Moreover, GABA (γ-aminobutyric acid), a biologically active compound which is related to the therapeutic effect of G. frondosa, is mainly detected in the mycelium but not in the fruiting body [11] (Table 3).
This entry is adapted from the peer-reviewed paper 10.3390/foods10010095
References
- Mayell, M. Maitake extracts and their therapeutic potential - A review. Alternative Medicine Review 2001, 6, 48-60.
- Mayuzumi, Y.; Mizuno, T. III. Cultivation methods of maitake (Grifola frondosa). Food Reviews International 1997, 13, 357–364.
- Montoya Barreto, S.; Orrego Alzate, C.E.; Levin, L. Modeling Grifola frondosa fungal growth during solid‐state fermenta-tion. Engineering in Life Sciences 2011, 11, 316–321.
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the pro-duction of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzyme and Microbial Technology 2004, 35, 369–376.
- Takama, F.; Minomiya, S.; Yoda, R.; Ishii, H.; Muraki, S. Parenchyma cells, chemical components of maitake mushroom (Grifola frondosa SF Gray) cultured artificially, and their changes by storage and boiling. In Proceedings of Proceedings of the Eleventh International Scientific Congress on the Cultivation of Edible Fungi, Sydney, Australia, 14–19 August 1981.
- Shih, L.; Chou, B.-W.; Chen, C.-C.; Wu, J.-Y.; Hsieh, C. Study of mycelial growth and bioactive polysaccharide produc-tion in batch and fed-batch culture of Grifola frondosa. Bioresource Technology 2008, 99, 785–793.
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. International Journal of Medicinal Mushrooms 2014, 16, 273–291, doi:10.1615/IntJMedMushr.v16.i3.80.
- Kurasawa, S.-i.; Sugahara, T.; Hayashi, J. Proximate and dietary fibre analysis of mushrooms. Nippon Shokuhin Kogyo Gakkaishi 1982, 29, 400–406.
- Muratsubaki, T.; Sayama, K.; Sato, K. Change of constituents in fruit body formation of Grifola frondosa. Nippon Shokuhin Kogyo Gakkaishi 1986, 33, 181–185.
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile taste components of several speciality mushrooms. Food Chemis-try 2001, 73, 461-466, doi:https://doi.org/10.1016/S0308-8146(00)00330-7.
- Huang, S.-J.; Tsai, S.-Y.; Lin, S.-Y.; Liang, C.-H.; Mau, J.-L. Nonvolatile taste components of culinary-medicinal Maitake mushroom, Grifola frondosa (Dicks.:Fr.) S.F. Gray. International Journal of Medicinal Mushrooms 2011, 13, 265-272, doi:10.1615/IntJMedMushr.v13.i3.60.
- Kawai, H.; Matsuzawa, M.; Tsutagawa, Y.; Sasaki, H.; Kasuga, A.; Aoyagi, Y. Relationship between fruiting bodies com-positions and substrate in Hiratake and Maitake mushrooms cultivated on sawdust substrate beds. Nippon Shokuhin Kogyo Gakkaishi 1994, 41, 419–424.
- Tabata, T.; Yamasaki, Y.; Ogura, T. Comparison of chemical compositions of Maitake (Grifola frondosa (Fr.) SF Gray) cul-tivated on logs and sawdust substrate. Food Science and Technology Research 2004, 10, 21–24.
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. Journal of Agricultural and Food Chemistry 2011, 59, 7841–7853.
- USDA. FoodData Central Search Results. Availabe online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169403/nutrients (accessed on 4 Janurary 2019).
- Miyazaki, T.; Yadomae, T.; Suzuki, I.; Nishijima, M.; Yui, S.; Oikawa, S.; Sato, K. Antitumor activity of fruiting bodies of cultured Grifola frondosa. Japanese Journal of Medical Mycology 1982, 23, 261–263.
- Ohno, N.; Suzuki, I.; Oikawa, S.; Sato, K.; Miyazaki, T.; Yadomae, T. Antitumor activity and structural characterization of glucans extracted from cultured fruit bodies of Grifola frondosa. Chemical and pharmaceutical bulletin 1984, 32, 1142–1151.
- Iino, K.; Ohno, N.; Suzuki, I.; Miyazaki, T.; Yadomae, T.; Oikawa, S.; Sato, K. Structural characterisation of a neutral anti-tumour β-d-glucan extracted with hot sodium hydroxide from cultured fruit bodies of Grifola frondosa. Carbohydrate re-search 1985, 141, 111–119.
- Ohno, N.; Adachi, Y.; Suzuki, I.; Sato, K.; Oikawa, S.; Yadomae, T. Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chemical & Pharmaceutical Bulletin 1986, 34, 1709–1715, doi:10.1248/cpb.34.1709.
- Nanba, H.; Hamaguchi, A.; Kuroda, H. The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (Maitake). Chemical & Pharmaceutical Bulletin 1987, 35, 1162–1168, doi:10.1248/cpb.35.1162.
- Hishida, I.; Nanba, H.; Kuroda, H. Antitumor activity exhibited by orally administered extract from fruit body of Grifola frondosa (Maitake). Chemical & Pharmaceutical Bulletin 1988, 36, 1819–1827, doi:10.1248/cpb.36.1819.
- Nanba, H.; Kubo, K. Antitumor substance extracted from Grifola. U.S. Patent 5,854,404, 29 December 1998.
- Kubo, K.; Aoki, H.; Nanba, H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biological and Pharmaceutical Bulletin 1994, 17, 1106–1110.
- Adachi, Y.; Okazaki, M.; Ohno, N.; Yadomae, T. Enhancement of cytokine production by macrophages stimulated with (1→ 3)-β-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biological and pharmaceutical bulletin 1994, 17, 1554-1560.
- Masuda, Y.; Kodama, N.; Nanba, H. Macrophage J774. 1 cell is activated by MZ-Fraction (Klasma-MZ) polysaccharide in Grifola frondosa. Mycoscience 2006, 47, 360–366.
- Lei, H.; Ma, X.; Wu, W. Anti‐diabetic effect of an α‐glucan from fruit body of maitake (Grifola frondosa) on KK‐Ay mice. Journal of Pharmacy and Pharmacology 2007, 59, 575–582, doi:doi:10.1211/jpp.59.4.0013.
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel hetero-polysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydrate Polymers 2016, 144, 382–389.
- Su, C.-H.; Lu, M.-K.; Lu, T.-J.; Lai, M.-N.; Ng, L.-T. A (1→ 6)-Branched (1→ 4)-β-d-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264. 7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. Journal of Natural Products 2020, 83, 231–242.
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. International Journal of Biological Macromolecules 2019, 121, 743–751.
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The positive effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. International Journal of Molecular Sciences 2019, 20, 5302.
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food and Chemical Toxi-cology 2019, 126, 295–302.
- Cui, F.; Zan, X.; Li, Y.; Yang, Y.; Sun, W.; Zhou, Q.; Yu, S.; Dong, Y. Purification and partial characterization of a novel anti-tumor glycoprotein from cultured mycelia of Grifola frondosa. International Journal of Biological Macromolecules 2013, 62, 684–690.
- Tsao, Y.-W.; Kuan, Y.-C.; Wang, J.-L.; Sheu, F. Characterization of a novel maitake (Grifola frondosa) protein that activates natural killer and dendritic cells and enhances antitumor immunity in mice. Journal of Agricultural and Food Chemistry 2013, 61, 9828–9838.
- Zhuang, C.; Kawagishi, H.; Preuss, H.G. Glycoprotein with antidiabetic, antihypertensive, antiobesity and antihyper-lipidemic effects from Grifola frondosa, and a method for preparing same. U.S. Patent 7,214,778. 8 May 2007.
- Gu, C.-Q.; Li, J.-W.; Chao, F.; Jin, M.; Wang, X.-W.; Shen, Z.-Q. Isolation, identification and function of a novel an-ti-HSV-1 protein from Grifola frondosa. Antiviral Research 2007, 75, 250–257, doi:https://doi.org/10.1016/j.antiviral.2007.03.011.
- Han, C.; Cui, B. Pharmacological and pharmacokinetic studies with agaricoglycerides, extracted from Grifola frondosa, in animal models of pain and inflammation. Inflammation 2012, 35, 1269–1275.
- Chen, S.; Yong, T.; Xiao, C.; Su, J.; Zhang, Y.; Jiao, C.; Xie, Y. Pyrrole alkaloids and ergosterols from Grifola frondosa ex-ert anti-α-glucosidase and anti-proliferative activities. Journal of Functional Foods 2018, 43, 196-205.
- Lin, J.-T.; Liu, W.-H. ο-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. Journal of Agricultural and Food Chemistry 2006, 54, 7564–7569.
- Yeh, J.-Y.; Hsieh, L.-H.; Wu, K.-T.; Tsai, C.-F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211.
- Sim, K.Y.; Liew, J.Y.; Ding, X.Y.; Choong, W.S.; Intan, S. Effect of vacuum and oven drying on the radical scavenging ac-tivity and nutritional contents of submerged fermented Maitake (Grifola frondosa) mycelia. Food Science and Technology 2017, 37, 131–135.
- Chang, S.-t.; Hayes, W.A. The biology and cultivation of edible mushrooms; Academic press: Cambridge, MA, USA, 2013.
- Ajlouni, S.O.; Beelman, R.B.; Thompson, D.B.; Mau, J.-L. Changes in soluble sugars in various tissues of cultivated mushrooms, Agaricus bisporus, during postharvest storage. In Developments in Food Science, Charalambous, G., Ed. Else-vier: Amsterdam, The Netherlands, 1995; Vol. 37, pp. 1865–1880.
- Yoshida, H.; Sasaki, H.; Fujimoto, S.; Sugahara, T. The chemical components of the vegetative mycelia of Basidiomy-cetes. Nippon Shokuhin Kagaku Kogaku Kaishi 1996, 43, 748–755, doi:10.3136/nskkk.43.748.
- Tsai, S.-Y.; Weng, C.-C.; Huang, S.-J.; Chen, C.-C.; Mau, J.-L. Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. LWT - Food Science and Technology 2006, 39, 1066-1071, doi:https://doi.org/10.1016/j.lwt.2005.07.017.
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food chemistry 2003, 82, 527–532.
