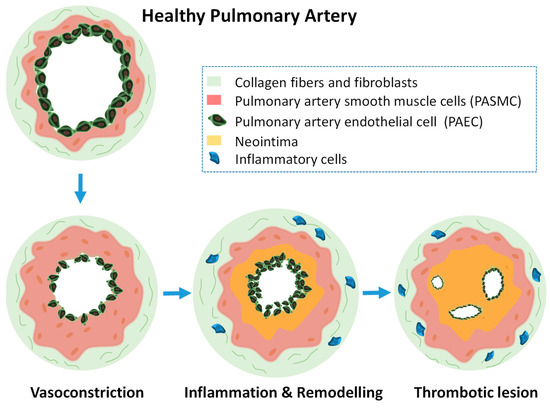
Pulmonary hypertension involves a continuous remodeling of the pulmonary vasculature, that is similar to cancer in some aspects due to the uncontrolled proliferation of certain cells. This leads to muscularization of pulmonary vessels, development of vascular lesions, continuous vasoconstriction, and final heart failure. Current pharmacological therapies only target three molecular pathways and as a result, patients can only improve their life quality but not without suffering adverse side effects. This fatal lung disease lacks effective treatments. Therefore, there are compelling reasons to find new molecular targets and novel therapies that reverse the development of the disease. In this context, miRNA-based therapies have shown promising results that will provided in the following text while explaining the important role that had played their nanoencapsulation.
- pulmonary hypertension,
- arteriopathy
- microRNA
- gene therapy
- nanoparticles
- smooth muscle cells
- endothelial cells
miRNA Targets in Pulmonary Hypertension
| miRNA | Model | Target Gene | Exp. 1 | Function | Ref. 2 |
|---|---|---|---|---|---|
| miR-9-1 + miR-9-3 |
PASMCs 3 from HMM 4 | HIF-1 α | ↑ | Phenotypic switch | [13] |
| miR-17-5p + miR-20a |
Bioinformatics +HEK293 cells | BMPR2 | ↑ | Differentiation, proliferation and fibrous matrix production of PAECS 5 and PASMCs | [112] |
| miR-100 | HMM, MCT 6 | mTOR | ↓ | Proliferation of PASMCs | [113] |
| miR-124 | HPAECs 7 | PTPB1 + PKM1/M2 | ↓ | Proliferation of PASMCs and PAFs 8 | [114] |
| miR-140-5p | Bioinformatics (miRBase database) | 23 genes & 7 signaling pathways | ↓ | Proliferation and pro-differentiation of PAECS, PASMCs & PAFs | [115] |
| miR-145 | HMM, HPASMC | BMPR2 | ↑ | Proliferation of PASMCs | [116] |
| miR-199a-5p | HPASMCs, HPAECs & HMM | SMAD3 | ↑ | Inhibit the level of NO and promote the concentration of Ca2+ | [117] |
| miR-204 | PAECs | TGFBR2, α-SMA, SMAD2/7 | ↓ | Proliferation and migration of PAECs | [118] |
| miR-205-5p | Hypoxic PASMCs & HMM | MICAL2 ERK1/2 signaling |
↓ | Proliferation of PASMCs | [110] |
| miR-206 | PASMCs & HMM | HIF-1 α /FHL-1 | ↓ | Promotion of cell entry into the S phase and PASMC proliferation | [119] |
| miR-214 | Hypoxic HPASMCs, Sugen/HMM | CCNL2, LMOD1, MEF2C, PTEN | ↑ | Proliferation of PASMCs by suppressing cell apoptosis | [120] |
| miR-339 | MCT murine model | FGF | ↓ | Proliferation of PASMC | [121] |
| miR-449a-5p | PASMCs from MCT | MYC | ↓ | Mitochondrial dysfunction and proliferation of PASMCs | [122] |
| miR-1281 | Hypoxic HPASMCs & MCT | HDAC4 | ↓ | Cell proliferation and migration | [123] |
| miR-637 | HPASMCs | CDK6 | ↓ | Increase PASMCs viability | [124] |
| miR-4632 | HPASMCs | JUN | ↓ | Inhibit proliferation and promote HPASMCs apoptosis | [125] |
miRNA-based therapies for Pulmonary Hypertension
| Targeted miRNA | NV 1 | Adm. 2 | Therapy | Effect | Application | Target cell | Ref. 3 |
|---|---|---|---|---|---|---|---|
| miR-17 | None (Antagomir) |
i.v.i. 4 | Inh. 5 | ↓Arteriopathy ↓RVP 6 ↓RVH 7 ↑Artery acceleration time |
MCT 8 HMM 9 |
PASMC | [128] |
| miR-21 | None (Antagomirs) |
i.v.i | Inh. | ↓Arteriopathy ↓RVP |
HMM | PASMC | [128] |
| miR-92a | None (Antagomirs) |
i.v.i | Inh. | ↓Arteriopathy | HMM | PASMC | [128] |
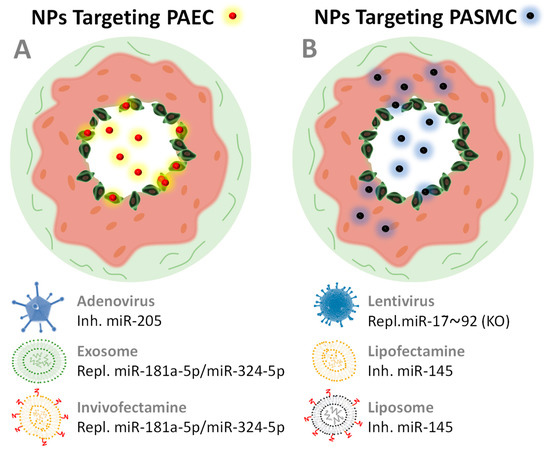
| miR-17~92 | Lentivirus (+ miR mimics) |
i.v.i. | Repl. | Restore hypoxia phenotype | PASMCs HM-KO-M 10 |
PASMC | [129] |
| miR-145 | Lipofectamine | s.c.i. 11 | Inh. | ↓RVP ↓ Arteriopathy |
HPASMC HMM |
PASMC | [116] |
| miR-145 | Liposomes 12 | i.v.i. | Inh. | ↓RVP ↓Artery thickness |
Sugen/HMM | PASMC | [7] |
| miR-181a-5p/miR-324-5p | EV and Invivofectamine | i.v.i | Repl. 13 | ↓RVP, ↓RVH ↓Cell proliferation ↓Angiogenesis |
HPAECs Sugen/HMM |
PAEC | [19] |
| miR-204a | Invivofectamine | int.neb. 14 | Repl. | ↓Cell proliferation ↓Vascular remodeling ↓PA blood pressure |
PASMCs MCT |
PASMC | [109] |
| miR-205 | Lipofectamine | in vitro | Repl. | ↓PASMC proliferation | PASMC HPASMC |
PASMC | [110] |
| miR-495 | Adeno-associated virus | i.v.i. | Inh. | ↓Vascular remodeling ↓Angiogenesis |
Sugen/HMM | PAEC | [130] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms21093253
References
- Stephen L. Archer; E. Kenneth Weir; Martin R. Wilkins; The Basic Science of Pulmonary Arterial Hypertension for Clinicians: new concepts and experimental therapies. Circulation 2010, 121, 2045-2066, 10.1161/CIRCULATIONAHA.108.847707.
- Rohit Budhiraja; Rubin M. Tuder; Paul M. Hassoun; Endothelial Dysfunction in Pulmonary Hypertension. Circulation 2004, 109, 159-165, 10.1161/01.cir.0000102381.57477.50.
- Jose L. Izquierdo-Garcia; Teresa Arias; Yeny Rojas; Victoria Garcia-Ruiz; Arnoldo Santos; Silvia Martin-Puig; Jesús Ruíz-Cabello; Metabolic Reprogramming in the Heart and Lung in a Murine Model of Pulmonary Arterial Hypertension. Frontiers in Cardiovascular Medicine 2018, 5, 110, 10.3389/fcvm.2018.00110.
- José Luis Izquierdo-García; Lucia Fadon; Marta Beraza; Unai Cossio; Jordi Llop; Jesus Ruiz-Cabello; In-vivo lung molecular imaging of choline metabolism in a rat model of pulmonary arterial hypertension. Pulmonary hypertension 2019, 54, PA1422, 10.1183/13993003.congress-2019.pa1422.
- Benoit Ranchoux; Lloyd Harvey; Ramon J. Ayon; Aleksandra Babicheva; Sébastien Bonnet; Stephen Y. Chan; Jason X.-J. Yuan; Vinicio A. De Jesus Perez; Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series). Pulmonary Circulation 2017, 8, 9999999999, 10.1177/2045893217752912.
- Benoît Ranchoux; Fabrice Antigny; Catherine Rucker-Martin; Aurélie Hautefort; Christine Péchoux; Harm Jan Bogaard; Peter Dorfmüller; S. Remy; Florence Lecerf; Sylvie Planté; et al. Endothelial-to-Mesenchymal Transition in Pulmonary Hypertension. Circulation 2015, 131, 1006-1018, 10.1161/circulationaha.114.008750.
- Marc Humbert; Hossein Ardeschir Ghofrani; The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2015, 71, 73-83, 10.1136/thoraxjnl-2015-207170.
- Hyung J. Chun; Sébastien Bonnet; Stephen Y. Chan; Translational Advances in the Field of Pulmonary Hypertension.Translating MicroRNA Biology in Pulmonary Hypertension. It Will Take More Than “miR” Words. American Journal of Respiratory and Critical Care Medicine 2017, 195, 167-178, 10.1164/rccm.201604-0886PP.
- Carlyne D Cool; Wolfgang Michael Kuebler; Harm Jan Bogaard; Edda Spiekerkoetter; Mark R. Nicolls; Norbert F Voelkel; The Hallmarks of Severe Pulmonary Arterial Hypertension: The Cancer Hypothesis - Ten years later.. American Journal of Physiology-Lung Cellular and Molecular Physiology 2020, _, _, 10.1152/ajplung.00476.2019.
- Ying Shi; Jianjun Huang; Jun Zhou; Ying Liu; Xiaodan Fu; Yimin Li; Gang Yin; Jifang Wen; MicroRNA-204 inhibits proliferation, migration, invasion and epithelial-mesenchymal transition in osteosarcoma cells via targeting Sirtuin 1. Oncology Reports 2015, 34, 399-406, 10.3892/or.2015.3986.
- A. Courboulin; R. Paulin; N. J. Giguere; N. Saksouk; T. Perreault; J. Meloche; E. R. Paquet; S. Biardel; S. Provencher; J. Côté; et al. Role for miR-204 in human pulmonary arterial hypertension. Journal of Cell Biology 2011, 192, _, 10.1083/jcb1924oia4.
- Wei Tao; Weiming Sun; Hailong Zhu; Jiayi Zhang; miR-205-5p suppresses pulmonary vascular smooth muscle cell proliferation by targeting MICAL2-mediated Erk1/2 signaling.. Microvascular Research 2019, 124, 43-50, 10.1016/j.mvr.2019.03.001.
- Fabo Shan; Junxia Li; Qing-Yuan Huang; HIF-1 Alpha-Induced Up-Regulation of miR-9 Contributes to Phenotypic Modulation in Pulmonary Artery Smooth Muscle Cells During Hypoxia. Journal of Cellular Physiology 2014, 229, 1511-1520, 10.1002/jcp.24593.
