Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Isoliquiritigenin (ISL), a natural bioactive compound with a chalcone structure, demonstrates high antitumor efficacy.
- isoliquiritigenin
- cancer
Thanks so much for your check. The entry will be online only after submission. (Please remove this note when submitting, thanks.)
1. Indroduction
ISL is a flavonoid with a simple chalcone structure. The structure of ISL and its metabolites are shown in Figure 1. The previous studies demonstrated the six metabolites detected in phase I [5,6,7], including liquritigenin (M1), 2′,4,4′,5′-tetrahydroxychalcone (M2), sulfuretin (M3), butein (M4), davidigenin (M5), and cis-6,4′-dihydroxyaurone (M6). Among the six metabolites, butein is the more active metabolite in the liver and in HT22 cells, with significant distribution on M1, M3, and M4 (Figure 1) [5,6,8]. Moreover, the previous study reported that the dominant metabolites of ISL are THC (2,4,2′,4′-tetrahydroxychalcone) and naringenin chalcone in lung cells [9]. In vivo absorption of ISL occurs in the intestines, transported to the liver for phase II biotransformation [7]. In phase II metabolism, liquiritigenin, glucuronidated ISL, glucuronidated liquiritigenin, and glucuronidated ISL are produced. Only glucuronidated liquiritigenin is predominant [10]. Many studies have suggested that secondary metabolites are involved in different biological activities and pharmaceuticals [5,7,8,11]. Therefore, these metabolites may differ in various cell lines or organs; however, they all share a similar structure to that of chalcone, which contains two aromatic rings connected by an unsaturated carbon chain, resulting in interconnected biological activities.
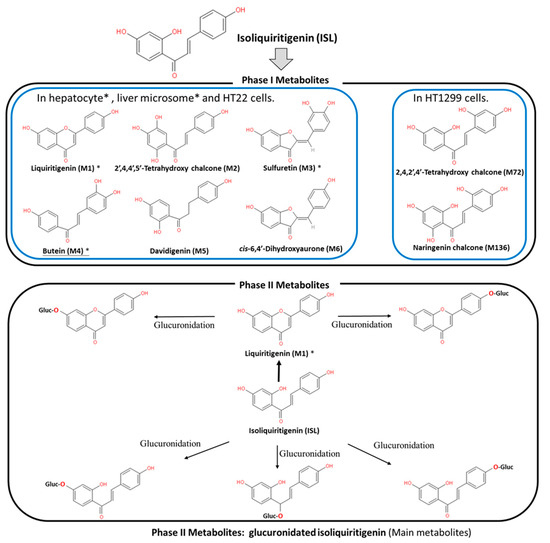
Figure 1. Metabolites of isoliquiritigenin (ISL). Phase I ISL metabolites were identified to be liquiritigenin (M1), 2′,4,4′,5′-tetrahydroxychalcone (M2), sulfuretin (M3), butein (M4), davidigenin (M5), and cis-6,4′-dihydroxyaurone (M6). Phase II metabolites were glucuronide conjugated process. Note: Figure was modified from [5,7,8].
2. ISL Pharmacokinetics
Evaluation of the safety of ISL is necessary for future clinical applications. Therefore, many studies, through different routes of administrations, including intravenously (IV), via hypodermic (IH) or intraperitoneal (IP) injection, and orally, have indicated that ISL exhibits a robust absorption capacity (absorption rate: ~60–90 min; oral absorption: >90%) with a strong elimination ability (t1/2: 2–4.9 h) [10,12,13,14]. Moreover, the data showed similar trends among different analytic methods, including high-performance liquid chromatography (HPLC), HPLC–MS/MS, and fluorescence spectrometry (SFS) [10,12,13]. This means that the absorption of ISL is quickly and widely distributed throughout the body [10,12,13,14]. Concentrations of ISL may vary in different tissues, including the heart, liver, lungs, spleen, kidneys, brain, muscles, and fat. ISL distribution mainly relies on the blood circulation, with the brain showing the lowest level of ISL due to the blood–brain barrier (BBB). These results imply that ISL is able to penetrate the BBB and exhibits neuroprotective activity in a male middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia rat model and high fat diet (HFD)-induced ICR mice model [15,16]. Interestingly, only after oral administration does [ISL]plasma exhibit a double-peak of ISL [14,17,18,19], the possible mechanism for which has been proposed as enterohepatic recycling. As a matter of fact, oral administration has become the most advanced application route.
3. ISL Nanoformulations and ISL Derivatives: Improved Efficacy
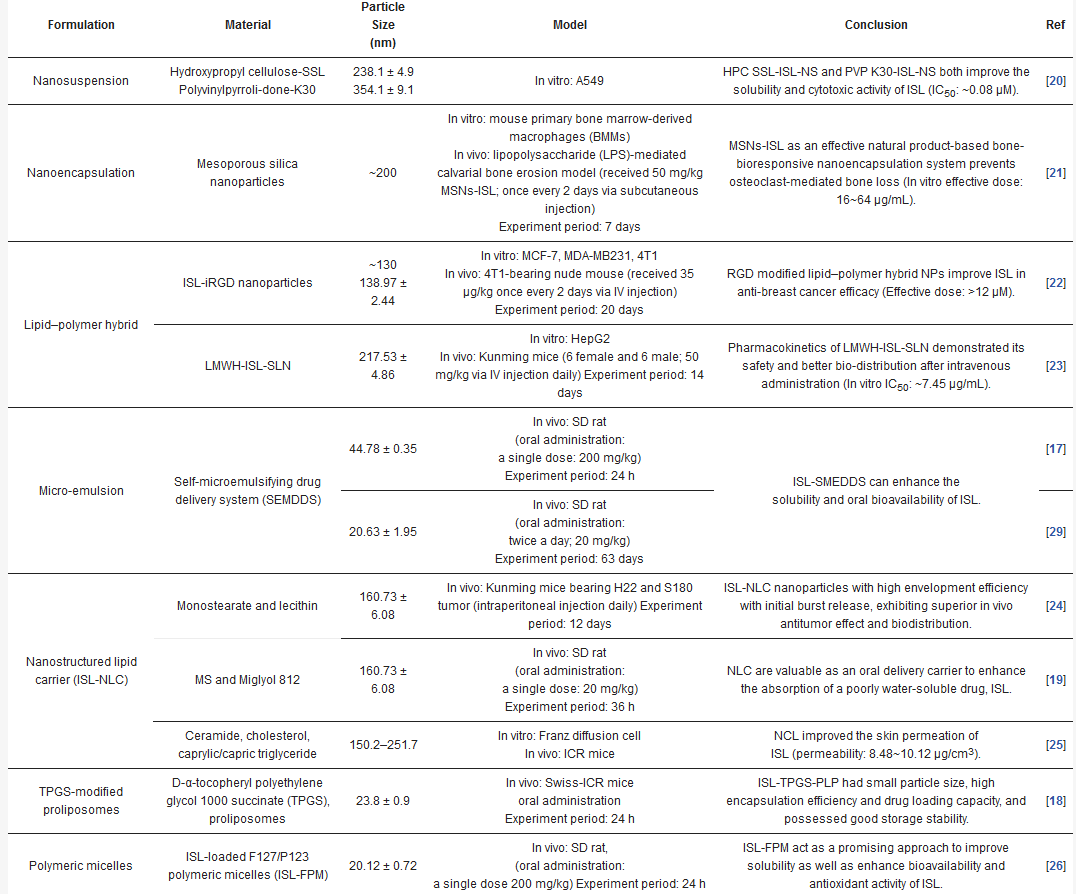
Generally speaking, poor bioavailability, rapid degradation, fast metabolism, and systemic elimination are the essential factors that lead to insufficient bioavailability. Insufficient bioavailability of ISL means that its efficacy is far less than 20% [10,14]. The term insufficient bioavailability implies that patients show intolerance to bulk administration of ISL to reach the desired effect, thereby highlighting the need to improve its effectiveness. To improve solubility, enhancing its bioavailability and distribution, encapsulated ISL nanoparticles or nano-ISL have been developed. Below, we summarize various ISL nanoparticles applied in preclinical studies, for example, polymer nanoparticles, liposomes, micelles, solid lipid nanoparticles (SLNs), and polymer conjugates.
-
Nanosuspension: ISL is milled with HPC (hydroxypropyl cellulose) SSL and PVP (polyvinylpyrrolidone) K30 to form a lamelliform or ellipse shape of the nanosuspension. HPC SSL and PVP K30 act as stabilizer. These two nanosuspension particles (size: 238.1 ± 4.9 nm with SSL; 354.1 ± 9.1 nm with K30) do not only improve the solubility issue, but also enhance the cytotoxicity a 7.5–10-fold [20].
-
Nanoencapsulation: Mesoporous silica nanoparticles (MSNs) are a solid material, acting as a biodegradable nanoscale drug carrier. When MSNs are encapsulated with ISL, they improve the efficacy of ISL in vitro and in vivo [21].
-
Lipid–polymer hybrid nanoparticle system:
- 3.1.
-
iRGD hybrid NPs: The composition of lipid–polymer hybrid nanoparticles (NPs) include lactic-co-glycolic acid (PLGA), lecithin, and a hydrophilic poly-ethylene-glycol (PEG). ISL-loaded hybrid NPs are composed of an inner PLGA core with an outer lipid layer (PEG, lecithin, and iRGD peptides). iRGD peptides (CRGDK/RGPD/EC, a tumor-homing peptides), can deliver drugs to a tumor. In vitro, ISL–iRGD NPs show stronger inhibition effects and induce apoptosis effects. In vivo, ISL–iRGD NPs show stronger effects in the viability of tumor cells. Herein, iRGD-modified lipid–polymer NPs showed better solubility, bioavailability, and targeting distribution [22].
- 3.2.
-
Hydrophilic polyanion solid lipid nanoparticles (SLNs): SLNs are composed of natural lipids such as lecithin or triglycerides that remain solid at 37 °C. SLNs can protect labile compounds from chemical degradation and can improve bioavailability. Low-molecular-weight heparins (LMWHs) are fragments of heparin showing hydrophilic polyanions that can improve the efficacy of ISL [23].
-
Microemulsion: The self-microemulsifying drug delivery system (SEMDDS) was designed for improving the solubility, absorption, and bioavailability of lipophilic drugs. The SMEDDS comprises ethyl oleate (EO; oil phase), Tween 80 (surfactant), and PEG 400 (co-surfactant). ISL-loaded SMEDDS has been proven to improve the solubility and oral in vivo availability [17].
-
ISL-loaded nanostructured lipid carriers (ISL-NLCs): NLCs mix solid lipids with spatially incompatible liquid lipids, which leads to a special nanostructure with improved properties for drug loading. ISL-loaded NLCs are constructed by glycerol monostearate (MS) and Mi-glyol-812 as the solid and liquid lipid materials to carry the ISL [24]. In pharmacokinetic studies, less than 10% of the NLCs remains in the stomach after oral administration, mainly absorbed in the colon [19]. Moreover, the antitumor effect of ISL-loaded NLCs has been evaluated in sarcoma 180 (S180)-bearing and murine hepatoma (H22)-bearing mice models via IP administration [24]. A biodistribution study showed that the ISL concentration of ISL-loaded NLCs in the tumor is higher 2.5-fold than free ISL. In a skin permeability study, the previous study suggested NLCs as a promising carrier to deliver the ISL [25].
-
TPGS-modified proliposomes: D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) has been selected as an excipient for ISL-loaded TPGS-modified proliposomes (ISL-TPGS-PLP), prepared using the film dispersion method with ISL-loaded proliposomes (ISL–PLP). ISL-TPGS-PLP can enhance the solubility, bioavailability and liver-targeting ability of ISL [18].
-
Polymeric micelles: PEO (polyethylene oxide)–PPO (polypropylene oxide)–PEO (polyethylene oxide) triblock copolymers are highly biocompatible and act as surface-active agents. P123 (PEO20–PPO65–PEO20) can remarkably enhance the retention of poorly soluble drugs in the blood circulation. Another important derivative of Pluronic, F127 (PEO100–PPO69–PEO100), possesses high biocompatibility. Therefore, mixed F127/P123 polymeric micelles have been developed, which have remarkably enhanced bioavailability with high encapsulation efficiency and low particle size. ISL-loaded F127/P123 polymeric micelles (ISL-FPM) improve the solubility as well as enhance the bioavailability and antioxidant activity of ISL [26].
-
Nanoliposomes (NLs): Drug-loaded PEGylated nanomaterials have shown effective cancer cell-killing ability, PEG2000-DPSE-QUE-NLs (polyethyleneglycol-2000-distearoyl phosphatidyl ethanolamine loaded with querce-tin (QUE)) can efficiently disperse in aqueous media compared to controls, and PEGylated (PEG2000-DPSE) NLs have been found to be effective drug delivery vehicles when simply loaded with ISL. ISL-NLs as tumor-targeted drug carriers are more effective in regulating glycolysis in colon cancer cell lines (CRC: HCT116) [27].
-
Hydrogel: Hydrogels are composed of hyaluronic acid (HA) and hydroxyethyl cellulose (HEC), and they can improve the skin permeation of ISL [28].
As described above, many experiments have been conducted to evaluate the various properties of ISL nanoformulation have been developed to address the problems of bioavailability and solubility. Nanoformulation studies have been conducted in vitro and in vivo (Table 1), demonstrating that ISL nanoformulations improve the bioavailability by 2–10-fold [17,24,26].
Table 1. Nano-formulation of ISL.
ISL-derived new compounds offer another solution to improve the bioavailability and water-soluble issues [31,32,33,34,35,36]. Considering the chalone structure, the α,β-unsaturated ketone is an important part of its biological activity by modifying on the phenol ring to improve the performance of ISL. We summarized a few new analogues of ISL in below (see Figure 2):
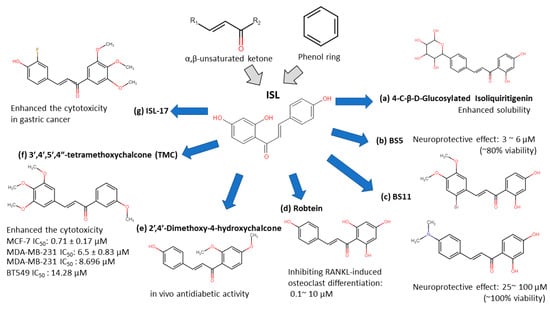
Figure 2. Isoliquiritigenin (ISL) derivatives.
However, the poor bioavailability and water-solubility issues remain in clinical applications. Future studies are still needed to elucidate the ISL formulations that would be more suitable for human clinical trials.
4. ISL Docking Model
ISL had been reported to exert diverse biological properties, but the specific molecular interaction that underlies these activities has not been fully unveiled. Based on molecular docking analysis, many studies have proposed that ISL has a direct interaction in different molecules (Figure 3), such as SIRT1 [38], VEGF2 receptor [39], GRP78 [40], FLT3 [41], EGFR [42], IKKβ [43], Toll-like receptors (TLRs) [44], CK-2 (IC50: 17.3 µM) [45], H2R [46], COX-2 [47], aromatase (Ki: 2.8 µM) [48,49], topoisomerase I [50] and DNMT1 [51]. These docking results imply that the binding pocket is composed of hydrophobic regions and is stabilized by a hydrogen bond with its neighboring carbonyl group. The hydrogen bond interactions and π–π stacking contribute to a tight interaction with the binding site. These docking results provide valuable information about the binding interactions of ISL and the active site, although more studies are required to approve them. Using a bioassay-guided purification method, suggested that isolated ISL acts as a xanthine oxidase inhibitor (IC50: 55.8 µM; Ki: 17.4 µM) to avoid transplantation rejection and ischemia reperfusion damage [52]. In brief, multiple docking candidates indicate that ISL exhibits multiple biological properties and serves as a potential lead compound for developing new therapy in cancer treatment.
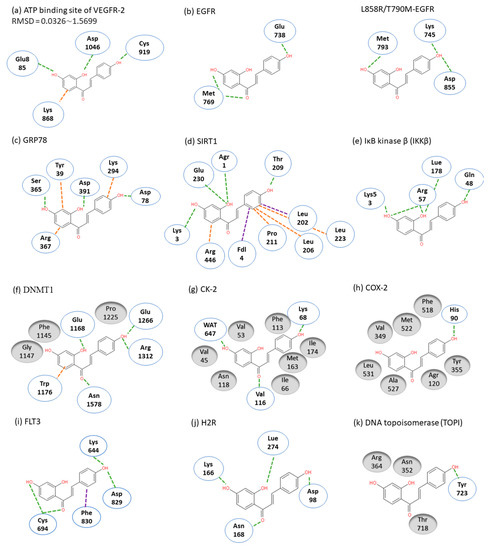
Figure 3. Molecular docking models. Interactions are represented in green (hydrogen bonding), orange (π–π stacking), purple (sigma-π) dash lines and gray (hydrophobic interaction: Van der Waals). (a) VEGFR-2; (b) EGFR; (c) GRP78; (d) SIRT1; (e) IKKβ; (f) DMNT1; (g) CK-2; (h) COX-2; (i) FLT3; (j) H2R; (k) TOPI.
2.5. ISL Biology Effects
In targeting cancers, ISL possesses various biologic activities, such as anti-inflammation, antioxidation, antiviral, antidiabetic, neuroprotective effect, chemopreventive, and antitumor growth properties (Figure 4 and Figure 5). A selective cytotoxicity effect of ISL has been reported (Table 2 and Table 3), and the effective dose in tumor cell lines shows very little cytotoxic effect on normal cells. Most studies have claimed that ISL significantly inhibits the viability of cancer cell but has little toxicity on normal cells. For example, Wu et al. (2017) compared the human endometrial stromal cells (T-HESCs; as a control) and human endometrial cancer cell lines (Ishikawa, HEC-1A, and RL95-2 cells). Their results indicated that ISL inhibits the growth of cancer cells at concentrations below 27 μM, but has little effect on normal cells [53]. Na et al. (2018) claimed that ISL shows little toxicity on normal hepatocyte cell lines (AML-12); only when applied in concentrations of over 100 μM is ISL harmful to normal hepatocytes [54]. Most studies have focused on the cytotoxicity between tumor and normal cells, and the effects of ISL on normal cells remain unknown. As Peng et al. (2015) mentioned, further research on the target organ toxicity or side effects of ISL is needed. The safety of ISL is always one of the most important concerns that must be evaluated.
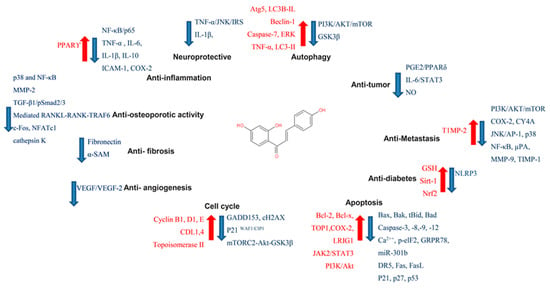
Figure 4. Pharmacological effect of ISL. The scheme presents the biological effects of ISL and molecular mechanisms of ISL against cancer via various signal pathways.
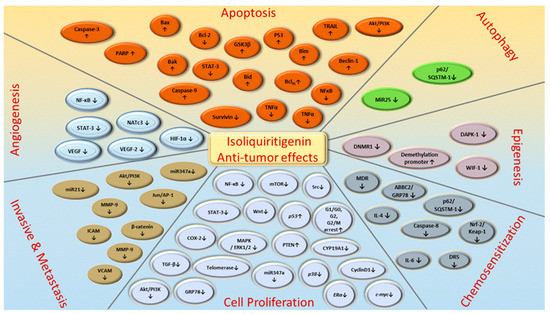
Figure 5. ISL-mediated regulation of molecular targets underlying anti-tumor effects, including tumor proliferation suppression, apoptosis induction, EMT/metastasis, epigenetic responses and sensitization to chemotherapy. Downward arrows (↓) represent downregulation while upward arrows (↑) represent upregulation. This figure was modified from [55].
Table 2. ISL influenced on normal cell lines.
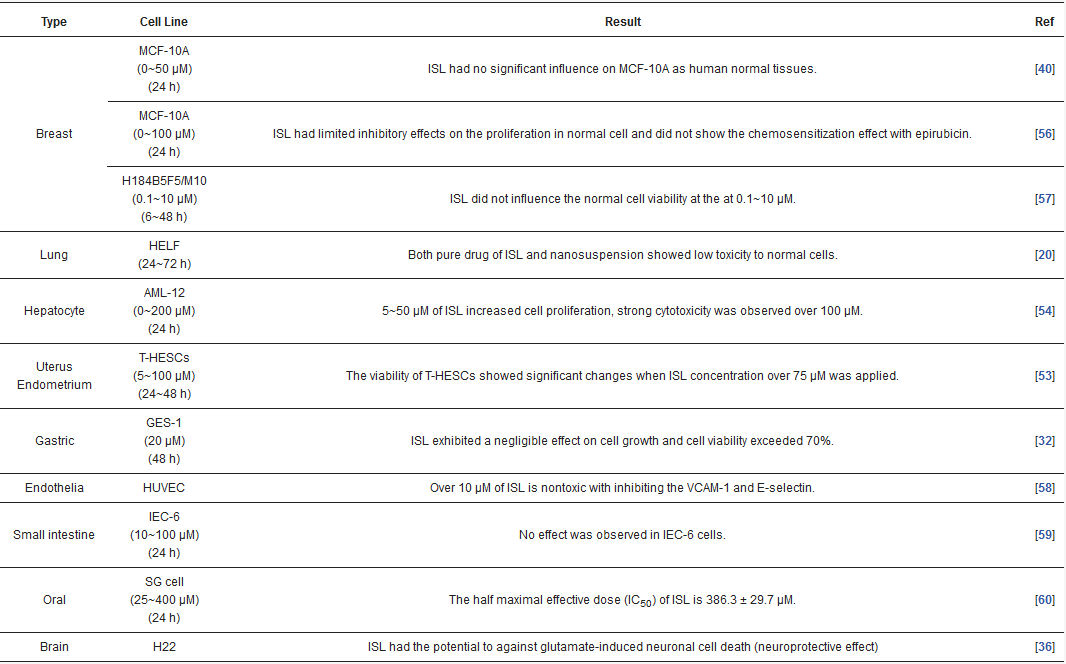
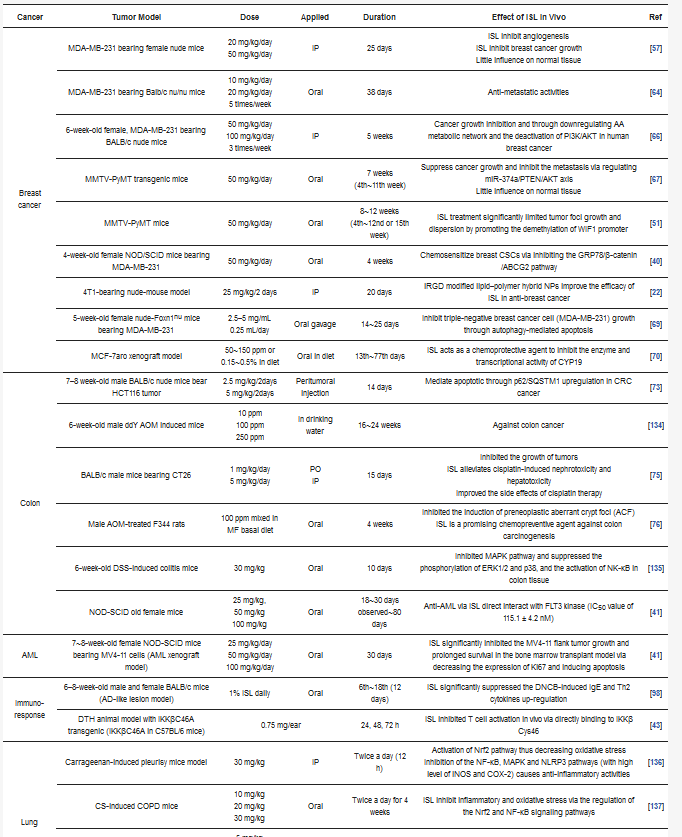
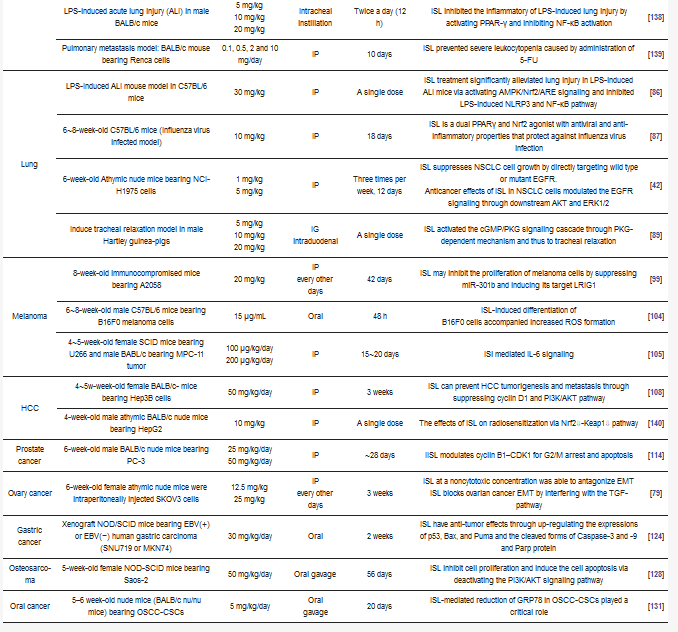
Table 3. Different pathways of various cancers regulated by ISL.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13010115
This entry is offline, you can click here to edit this entry!
