G protein-coupled receptor (GPCR) kinases (GRKs) play an important role in the regulation of signaling of GPCRs that bind neurotransmitters. The canonical model of GPCR desensitization posits that GRKs phosphorylate ligand-activated GPCRs, and this phosphorylation prepares receptors for the high-affinity binding of arrestin proteins. Arrestin binding prevents further G protein coupling, promotes receptor internalization, and initiates and/or facilitates specific branches of signaling. Existing data suggest that the role of GPCR phosphorylation by GRKs is distinct in different receptors. The relationship between G protein- and arrestin-mediated signaling on the one hand, and therapeutic and side effects of drugs on the other, is more complex than is widely believed. Also, the relationship between rapid (minutes to hours) GRK/arrestin-mediated regulation and long-term (days to weeks) neural plasticity remains to be elucidated.
- GRK
- GPCR
- neurotransmitter
- arrestin
1. Introduction: GRKs in GPCR Signaling and Trafficking
Activation-dependent phosphorylation of rhodopsin was discovered in the early 1970s [1,2], long before it became clear that rhodopsin belongs to the family of G protein-coupled receptors (GPCRs). Comparison of the primary structure and membrane organization of rhodopsin [3] and β2-adrenergic receptor (β2AR) [4] demonstrated that both belong to the same protein family, now known as the GPCR superfamily, of which humans express ~800 different subtypes. These receptors are also called 7TMRs, as all GPCRs contain seven transmembrane α-helices. Rhodopsin kinase (modern systematic name GRK1, which stands for G protein-coupled receptor kinase 1 [5]) was the first GPCR kinase (GRK) discovered. It was shown to selectively bind and phosphorylate light-activated rhodopsin [6,7]. Later, the Kuhn group demonstrated that rhodopsin phosphorylation is necessary to quench its signaling [8]. In the same year, the Lefkowitz group reported that β2AR is phosphorylated by a cAMP-independent kinase and that this phosphorylation facilitates receptor desensitization [9]. As this kinase was discovered via its ability to phosphorylate β2AR, when cloned, it was called β2-adrenergic receptor kinase, or βARK for short (modern systematic name GRK2) [10]. GRK2 was also shown to phosphorylate rhodopsin in a strictly activation-dependent manner, exactly like rhodopsin kinase [11], suggesting that both GRKs are specific for active GPCRs. When rhodopsin kinase was cloned [12], it was found to have a structure similar to that of the kinase that phosphorylates agonist-activated β2AR [10]. The mechanism underlying the specificity of GRKs for active GPCRs was subsequently established: the activation of GRK1 required physical interaction with the active rhodopsin [13]. The same activation mechanism was demonstrated for non-visual GRKs with β2AR [14] and D2R [15].
Rhodopsin phosphorylation was shown to increase the binding of a 48-kDa protein [16] (later termed visual or rod arrestin; systematic name arrestin-1). The binding of this protein was shown to be necessary to stop rhodopsin coupling to its cognate G protein, transducin [8]. It turned out that highly purified β2AR kinase has a limited effect on β2AR coupling to its cognate G protein, Gs, suggesting that a non-visual arrestin homologue is necessary for desensitization [17]. Both arrestin-1 [18] and its non-visual homologue [19] were subsequently cloned. These proteins are highly homologous. However, arrestin-1 and its homologue demonstrated clear preference for rhodopsin and β2AR, respectively [20]. Therefore, the non-visual protein was originally termed β-arrestin (systematic name arrestin-2).
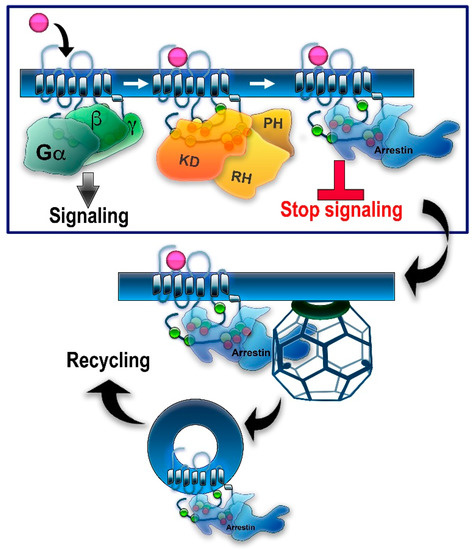
In genetically modified mice, it was shown that the absence of either GRK1 [21] or arrestin-1 [22] results in abnormally prolonged rhodopsin signaling. These and other findings resulted in the general model of two-step homologous (specific for the receptor that was activated) GPCR desensitization: the active receptor is phosphorylated by a GRK (seven GRK subtypes are expressed in most vertebrates [5,23]), whereupon arrestin (four subtypes are expressed in most vertebrates [24]) binds to it and stops the signaling by direct competition with the G protein [25,26] (reviewed in [27]) (Figure 1). Non-visual arrestins bound to phosphorylated GPCRs were shown to directly interact with the key components of the internalization machinery of the coated pit, clathrin [28], and clathrin adaptor AP2 [29], so that GPCR phosphorylation and subsequent arrestin binding promote receptor endocytosis. While the role of both GPCR phosphorylation and arrestin binding actually differs for various receptor subtypes (Figure 2), the simplified general paradigm posits that GRKs play a critical role in the two processes that reduce cell responsiveness: precluding G protein coupling and facilitating receptor removal from the plasma membrane. These are the key GRK effects on the signaling of most GPCRs, including the neurotransmitter receptors belonging to the GPCR family that are discussed below. The amount of information available regarding the effects of GRK phosphorylation, particularly in biologically relevant in vivo models, varies significantly for different receptors. While several independent studies have been performed with opioid receptors, including the use of knockin mice expressing receptor mutants where some or all potential GRK phosphorylation sites were eliminated, other neurotransmitter GPCRs have been studied much less comprehensively. As this review is based on experimental evidence, opioid receptors will be discussed in greater detail than other GPCRs that bind neurotransmitters.
2. From Neurotransmitter Receptor Regulation to Neural Adaptation: The Role of GRKs
It is not difficult to imagine that altered availability and/or function of GRKs, leading to impaired receptor desensitization/trafficking, would result in enhanced or reduced responsiveness to agonists, as in cultured cells. However, chronic treatment with neurotropic drugs often results in long-term neural plasticity, altering the responsiveness to these drugs, which develops over hours or days, in contrast to the much faster timescale of receptor phosphorylation and arrestin recruitment, both of which occur within minutes in cultured cells. This long-lasting plasticity is initiated by agonist stimulation of the neurotransmitter receptors, which would initially engage GRKs, and it is a legitimate—and intriguing—question whether GRK-dependent phosphorylation of the neurotransmitter receptors plays a role in the development of long-term neural plasticity.
2.1. GRKs in the Regulation of Acute Responsiveness to Neural Stimulation
2.2. GRK-Mediated Rapid Desensitization in Long-Term Neural Adaptations
2.3. Neurotropic Drugs: To Bias or Not to Bias?
All therapeutically active drugs have side effects. Since the discovery of arrestin-mediated signaling by GPCRs, the idea of biased signaling, i.e., signaling engaging only one branch of the GPCR pathway, either G protein or arrestin, has gained popularity. For the biased signaling to be possible, the two branches, the G protein- and arrestin-mediated, should be independent, and originally they were believed to be. There are obvious limitations to this independence. The fact that GRKs 2 and 3 use two products of G protein activation, Gβγ [60,61,62] and activated Gαq/11 [101], for membrane localization suggests that G protein activation likely plays a role in GPCR phosphorylation by these GRKs, which is often necessary for arrestin binding. This might limit what arrestins can do in the absence of G protein activation. On the other hand, GRKs 4/5/6 have C-terminal lipid modifications and/or specific sequences mediating their attachment to the membrane independently of G proteins (reviewed in [5]). Thus, phosphorylation of the same GPCRs by GRKs 5/6 instead of GRKs 2/3 might bypass the need for G protein and enable arrestin recruitment and arrestin-mediated signaling. Recently, a question arose as to whether G protein activity is required for arrestin-mediated signaling in some capacity other than for the GRK recruitment to the receptor. It has been reported that the presence of functional G proteins is indispensable for arrestin-mediated activation of the ERK pathway [165]. In cells, the ERK pathway is activated via G protein as well as arrestin-dependent mechanisms [166,167], but it can be activated exclusively via G proteins independently of arrestins [168]. Whether the reverse is true has not yet been unambiguously determined (see short discussion in [169]). The issue of the potential and limitations of G protein vs. arrestin bias of GPCR ligands was recently discussed in depth [148].
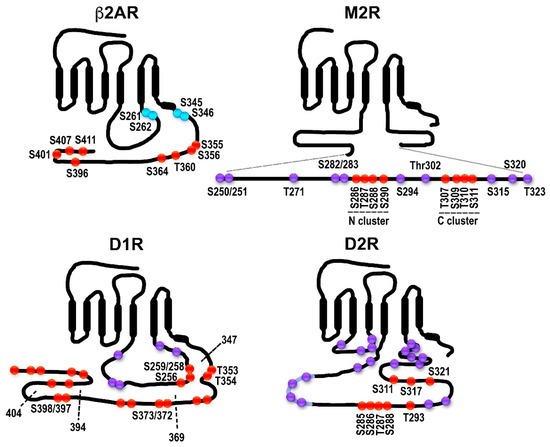
On the physiological side, the idea is based on the notion that the therapeutic action might be mediated by one signaling branch and the side effects by another. The attraction of this theory is obvious but the experimental basis is limited (discussed in [148]). Arguably, nowhere has this theory received more attention than in the field of opioid therapy. Opioid drugs remain the mainstay of pain management. Their utility is limited, however, not only by the tolerance that develops upon long-term use but also by multiple unwanted side effects including life-threatening respiratory depression, gastrointestinal disturbances, and addiction. Since GPCRs signal via both G protein- and arrestin-mediated pathways, it has been suggested that the therapeutic antinociceptive effects of opioid drugs are mediated by G proteins, whereas arrestin-dependent signaling is responsible for the side effects. This notion is based on studies of G protein-biased opioid agonists such as PZM21 [170] and TRV-130 [171]. TRV130 induced less MOR phosphorylation and arrestin recruitment than unbiased agonists. It is a potent analgesic with less evident gastrointestinal dysfunction and respiratory suppression than morphine [171,172]. However, direct proof that the arrestin-mediated signaling that takes place following MOR phosphorylation and arrestin recruitment is responsible for the opioid side effects is lacking. It is important to note that in many cases—clearly in the case of opioid receptors, for which phosphorylation by GRKs is a prerequisite for arrestin recruitment—bias for or away from arrestin is in reality a bias for or away from specific GRKs. What hampers the meaningful discussion of biased signaling is the lack of information on the phosphorylation patterns of the most relevant receptors and their potential barcoding by GRK isoforms. A recent important study using knockin mice expressing phosphorylation-deficient MOR with all or nearly all phosphorylation sites eliminated demonstrated that the side effects of both morphine and fentanyl were, if anything, exacerbated [158]. This effect was accompanied by enhanced analgesia and diminished tolerance. Furthermore, the EC50 values for morphine analgesia and respiratory depression/constipation across lines with different phosphorylation levels are highly correlated (R2 > 0.9), suggesting that these effects are not independent. These experiments strongly suggest that all major physiological effects of MOR activation, both good and bad from our perspective, are largely mediated by G proteins.
This entry is adapted from the peer-reviewed paper 10.3390/cells10010052
