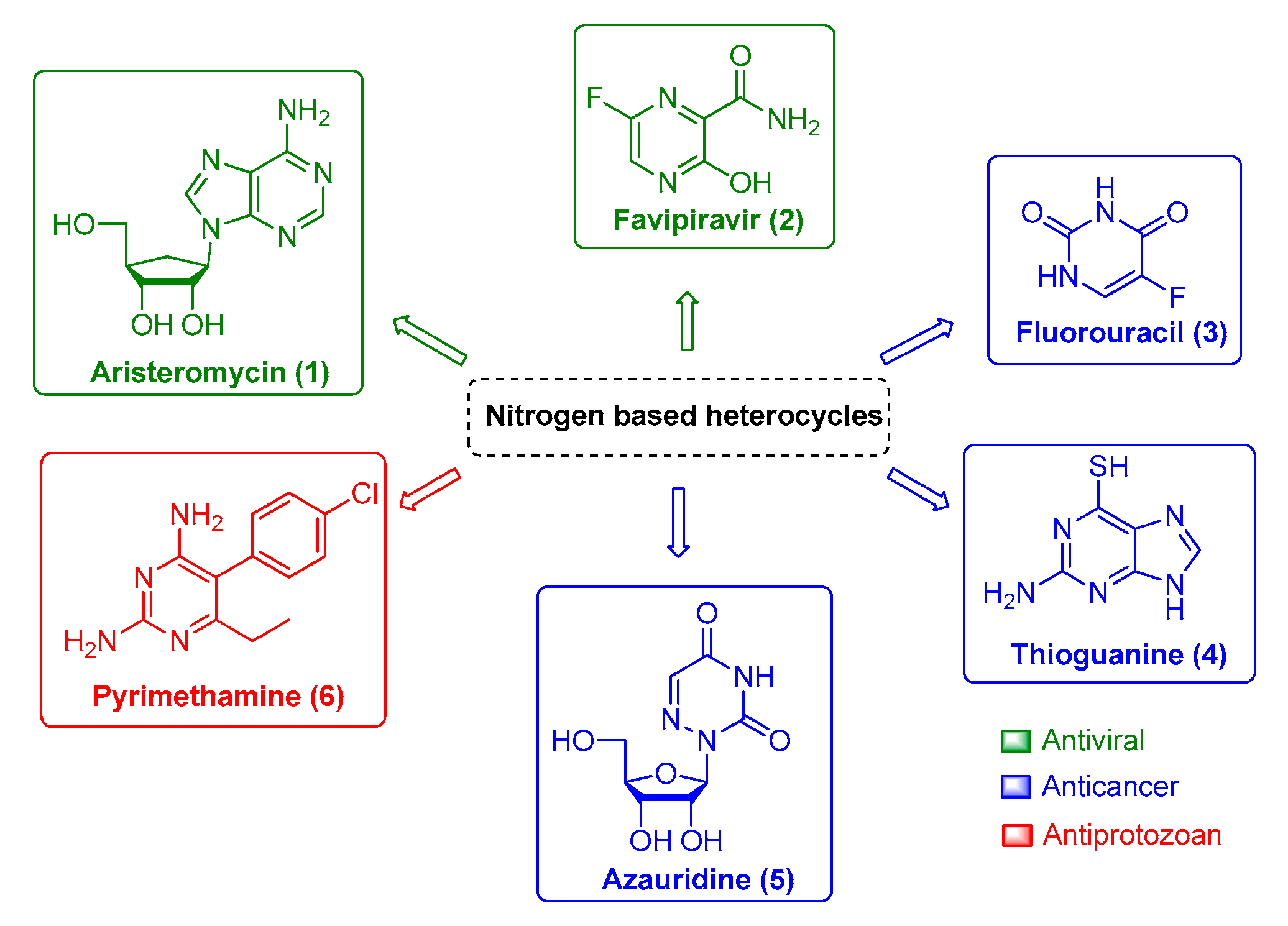
Arboviruses, in general, are a global threat due to their morbidity and mortality, which results in an important social and economic impact. Chikungunya virus (CHIKV), one of the most relevant arbovirus currently known, is a re-emergent virus that causes a disease named chikungunya fever, characterized by a severe arthralgia (joint pains) that can persist for several months or years in some individuals. Until now, no vaccine or specific antiviral drug is commercially available. Nitrogen heterocyclic scaffolds are found in medications, such as aristeromycin, favipiravir, fluorouracil, 6-azauridine, thioguanine, pyrimethamine, among others. New families of natural and synthetic nitrogen analogous compounds are reported to have significant anti-CHIKV effects. In the present work, we focus on these nitrogen-based heterocyclic compounds as an important class with CHIKV antiviral activity.
- Nitrogen-Based Heterocyclic Compounds,Arboviruses
1. Introduction
Mosquito-borne viruses represent a serious health problem worldwide due to a number of factors, including the vectors’ growing geographic expansion and the easy mobility of humans across all continents, which culminates in a perfect scenario for virus propagation [1]. Among the arboviruses that cause human disease, Chikungunya virus (CHIKV) is one of the most relevant, mainly because of its great social and economic impact resultant from its high morbidity, although it is rarely associated with death. It was first isolated in the 1950s in the region currently known as Tanzania, and since then has been responsible for some sporadic outbreaks in the African and Asian continents [2]. CHIKV is transmitted by mosquitos of the genus Aedes spp., most commonly by Aedes aegypti and Aedes albopictus [3].
The adaptation of CHIKV to replicate in Aedes albopictus has expanded its spread around the world, resulting in outbreaks not only in Africa and Southeast Asia, but in other continents such as Europe (mainly in Italy and France) and in the Americas [4]. In the Americas, the first CHIKV outbreak was reported in 2013 on the Caribbean island of Saint Martin, which resulted in more than 1.2 million cases [5]. Since then, other countries such as Brazil, have documented persistent outbreaks of CHIKV. In the second half of 2020 alone, approximately 81,000 cases of chikungunya fever have been reported across the Americas, and 97% of the cases were in Brazil [6,7].
CHIKV is a member of the Togaviridae family and of the Alphavirus genus, and is contained in the Semliki forest antigenic complex, which has other alphaviruses, such as: Ross River, O’nyong-nyong, Getah, Bebaru, Semliki forest, and Mayaro viruses, which are endemic in South America [7]. Clinical symptoms are observed in almost all infected individuals and more than half of the patients have persistent polyarthritis, which might last several months or years after the acute viral infection [8].
Despite causing morbidity, there is no therapeutic treatment available on the market against CHIKV. Some compounds with a broad antiviral activity such as ribavirin, harringtonine, and interferon-alfa (IFN-α) showed great efficiency against CHIKV only in vitro [9]. On the other hand, the use of chloroquine exhibited a dose-dependent and time-dependent inhibitory effect on CHIKV replication in vitro, but clinical studies have failed to prove its effectiveness in infected patients [10,11].
In clinical practice, treatment is strictly limited to symptom relief, with the use of antipyretics, analgesics, corticosteroids, and non-steroidal anti-inflammatory drugs. In some patients with severe arthralgia and polyarthritis, the administration of methotrexate and sulfasalazine which are called disease modifying anti-rheumatic drugs, is necessary [12].
Therefore, it becomes clear that there is an urgency and a necessity to discover new classes of compounds that can be used in clinics to treat CHIKV infection. In the search for new candidates with anti-CHIKV activity, nitrogen-based heterocyclic derivatives, such as pyrimidine, pyrazine, and purine, have emerged as promising compounds. Scientific literature points to a very interesting potential of these scaffolds against CHIKV infection therapy.
CHIKV Infection
CHIKV has a single-strand, positive-polarity RNA genome, whose size varies between 9.7 and 12 kb [13]. The viral particles, which are often spherical, have a diameter of 70 nm and an isometric nucleocapsid of 40 nm [14]. The proteins are encoded by two open reading frames (ORFs) in which the first two-thirds of the genome from the 5′ end is responsible for expression of the non-structural proteins (NSPs) named nsP1, nsP2, nsP3, and nsP4; whereas the last one-third located at the 3′ end codifies a subgenomic RNA that originates from three structural proteins and two peptides: capsid (C), enveloped glycoprotein 1 (E1), glycoprotein enveloped 2 (E2), E3, and 6K [1,15,16].
Glycoproteins E1 and E2, which form heterodimers, are found on the viral surface and are involved in the target cell recognition [10,12,17]. After interaction with a specific receptor in the cell membrane, the virus particle undergoes endocytosis in vesicles covered by clathrins. The pH acidification in the endosome causes a tridimensional rearrangement of the E1 subunit culminating in the exhibition of the fusion peptide, which will be responsible for the fusion of the envelope with the endosomal membranes and the consequent release of the nucleocapsid to the cytoplasm. Thereafter, the RNA genome gets exposed, and translation of NSPs begins. These NSPs are responsible for the replication complex assembly and perform all the steps necessary for successful virus replication. It is important to highlight that the NSPs are translated as a polyprotein that are further processed as nsP1, nsP2, nsP3, and nsP4 by the action of the viral protease nsP2 [10,13].
The nsP1 manages the enzymatic activities of guanine-7-methyltransferase and guanylyl transferase. It is also associated with membranes and probably acts as a scaffold to attach the viral replication complex (non-structural proteins) to the host membranes. The capping of viral genomes is critical because it has its importance in translating proteins from genomic and subgenomic RNAs, making it a potential target for antiviral drugs because it is specific and less prone to side effects [11,18,19].
NsP2 has the following enzymatic functions: RNA helicase, nucleoside triphosphatase (NTPase), and RNA-dependent RNA-triphosphatase-5′RNA activity, despite its protease activity. This protein is essential for viral viability [16,20]. The nsP3 macrodomain acts as part of the replicase unit and is present in RNA synthesis. It is subdivided into three domains: N-terminal that connects with negatively charged polymers, the one unique to alphavirus (AUD) and hypervariable C-terminal (HVR), which are responsible for the metabolism of the ADP ribose derivatives, which regulate functions in the cell. NsP4 acts as an RNA-dependent RNA polymerase that synthesizes new viral RNAs from the pre-existing RNA genome [3,12,18,21].
The lack of proofreading activity of the nsP4, which is responsible for high mutation rates [22], in association with the A226V substitution at E1 protein, may be related to a greater virus virulence and allow its best adaptation to replicate in Aedes albopictus mosquitoes, which explains the rapid spread of the disease between 2004 and 2010 to many countries around the Indian Ocean [23]. To date, four CHIKV genotypes have been described: Asian, East Indian, West African, and East/Central/South African. The Asian genotype was first detected in the Caribbean region in late 2013 and then spread throughout Central America [14].
2. CHIKV Drugs
The ideal treatment of CHIKV-infected patients needs a safe, specific, and efficient antiviral drug that could both eliminate viral replication and block systemic and joint inflammation due to viral infection. Drugs commercially available for other diseases including fever-like illnesses are often used to manage CHIKV symptoms, such as orlistat, chloroquine, favipiravir, arbidol, and ribavirin. However, due to lack of specificity of these drugs against CHIKV infection, they fail to promote complete viral clearance and abrogate its clinical manifestation [8].
The development of a wide spectrum antiviral drugs against different arboviruses, including CHIKV, was for a long time encouraged by the fact they share several mechanisms of pathogenicity. In practice, this strategy has proven quite challenging, since each virus has different cell tropism, which elicits distinct inflammatory responses, as well as promotes different mechanisms of immune evasion. The structural and functional diversity of viral enzymes often used as pharmacological targets, such as the RNA-dependent RNA polymerase (nsP4) and protease (nsP2), significantly alters the affinity and activity of polymerase or protease inhibitors, respectively, which, in the end, drives the antiviral development to a virus-specific pathway [10,13,24,25,26,27,28,29,30].
Therefore, finding potent and efficient antivirals with novel scaffolds that have the potential to specifically interact and impair viral enzymatic activities of a single viral family or viral genus is the pursued path toward successful drug development. In this context, the nitrogen-based heterocyclic analogs are an important class of organic compounds that have a privileged position. They are a valuable source for therapeutic agents, which is corroborated by the increasing attention received by these compounds over the last decades. Among them, pyrimidine and its derivatives have received great attention due to their biological and pharmacological activities against viral infection, such as aristeromycin (1) and favipiravir (2); as well as anticancer drug, such as fluorouracil (3), thioguanine (4), and azauridine (5); and as antiprotozoal drugs, such as pyrimethamine (6) (Figure 1). Moreover, this class of molecules is usually reported as being druggable and safe for human use, which makes it more attractive for further development. Therefore, in the present review, we will summarize natural and synthetic nitrogen-based heterocyclics and their derivatives with reported anti-CHIKV activity [9,11,19,25,31,32,33,34,35,36,37,38,39] and explore the potential of these molecules as an interesting class for antiviral drug development.
Nitrogen Heterocycle–Coumarin Hybrid Compounds
Several natural compounds based on herbs and plant extracts were investigated for activity against CHIKV [40,41]. Among natural products, coumarin has gained much attention due its pharmacological properties against several diseases. Coumarins with biological activities can be found in various parts of the plant, as seeds, leaves, and roots and also in distinct plant families, such as Apiaceae, Rutaceae, Clusiaceae, and Umbelliferae [42]. Natural coumarin derivatives have been described as having anticoagulant [43], antibacterial, antioxidant, and anticancer properties [44].
Coumarin has been known to play an incremental role in targeting various cell pathways, inhibiting growth and replication, among other targets for a wide range of viruses. The anti-influenza activity of coumarin derivatives was associated with neuraminidase’s inhibition of the viral envelope protein [45]. In the case of the human immunodeficiency virus (HIV), coumarin analogs, isomesuol and mesuol (extracted from Marila pluricostata), could act via the phosphorylation of p65 (NF-kB) [46]. While in the hepatitis C virus (HCV), coumarins could act against its replication, increasing the interferon-mediated antiviral responses [47]. The activity of coumarins against dengue virus (DENV) has already been reported, but its mechanisms of action requires further investigation; however, initial studies point to the NS2B/NS3 complex as a possible coumarin target [48]. The activity of coumarin against CHIKV is still under investigation [42,49,50,51,52,53].
In Figure 2, we have summarized some coumarin–nitrogen-based heterocyclic derivatives that were reported to have anti-CHIKV activity. Compounds 7, 8, 11, 14 and 16 were the most potent inhibitors, with EC50 values ranging from 10.2 to 19.1 μM (concentration necessary to reduce 50% of viral infection in Vero cells), and with a selectivity index (SI) around 5.6 and 11.5. The calculation of the molecular lipophilicity quantified by logP is a major determinant for the absorption of the compound, distribution in the body, penetration through biological barriers, metabolism and excretion (ADME). An ideal logP range is generally between 0.4 and 5.0. Higher values are indicative of malabsorption or permeation of the components. For these pyrimidine–coumarin hybrids, the logP values remained in the range from 2.74 to 3.57 [34,36].
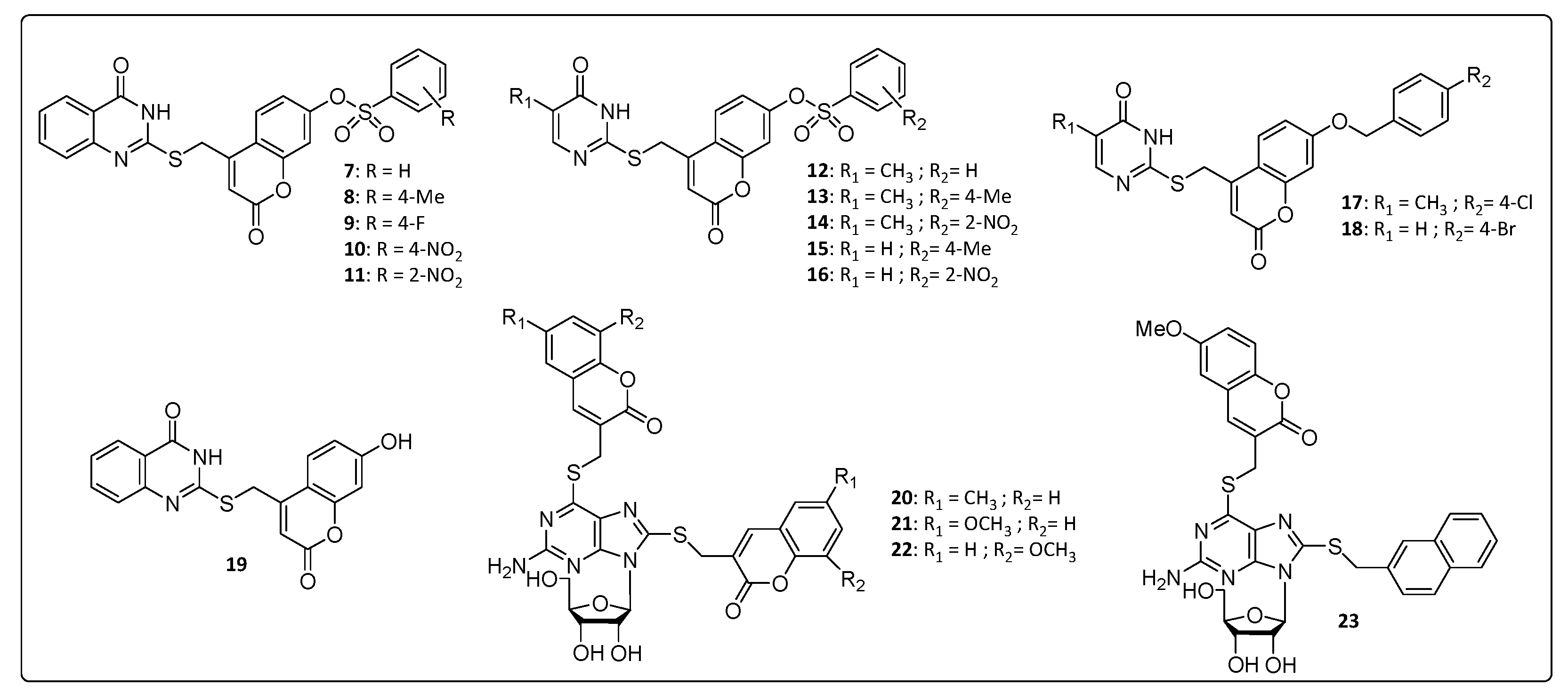
With respect to the benzenesulfonyl ring on coumarin, the para-methyl group at 8, increased the potency and SI value when compared to that of the unsubstituted derivatives 7. The same correlation can be done with nitro compound 11 that increased activity by 2 times, approximately, compared to the 4-position for the same substituent in 10. While for pyrimidine analogs, 12–16, the nitro group at 2-position on the benzenesulfonyl ring on coumarin showed the best activity. Overall, the quinazoline derivatives, 7–11, were more active than the pyrimidine analogs, 12–16, whose logP values are directly proportional to the activity presented 8 > 13 > 15 and 11 > 14 > 16. The presence of hydrophobic substituents at the 5 and 6 position of pyrimidine nucleus (7–11) was an important synthetic strategy, conferring greater activities against CHIKV and other viruses, such as HIV and HCV [34].
A series of thioguanine-coumarins derivatives were studied, and the methoxylated coumarins [54] (21, 22 and 23) exhibited the best activities against CHIKV compared to the benzouracil coumarins (9–19). EC50 values were below 13.9 μM with a significant selectivity window covering SI values between 9.37 and 21.7, whereas the molecular lipophilicity of 20, 21, 22 and 23 were between 2.97 and 3.77. The replacement of the coumarinyl (21) by naphthyl group (23) resulted in no significant influence in the EC50 value, probably because of the similar size and planarity of the two substituents. In contrast, substitution of the naphthyl to benzyl (not shown) considerably decreased the EC50 values [54], confirming the importance of the size and planarity of the substituents for proper antiviral activity [54].
This entry is adapted from the peer-reviewed paper 10.3390/life11010016
