Consumption of pigmented rice as a staple food is rapidly increasing due to their healthy prospective and considered as functional food ingredients. Greater interest has been shown in many color rice varieties due to their multiple biological activities. The phenolic compounds have been found to consist of anthocyanidins, ferulic acid, diferulates, anthocyanins and polymeric proanthocyanidins. Anthocyanin is located in the bran layers of the rice kernel, while phenolic acids are mainly present in the bran layers of rice, existing as free, conjugated and bound forms. Keeping in view the several health benefits associated with the functional ingredients, such as anti-inflammatory, antioxidative and anticancer effects, pigmented rice is considered as a functional food and food ingredient in many Asian countries.
- pigmented rice bran
- medicinal values
- bioactive com
1. Introduction
Rice is the second-largest cereal grain grown after maize and is consumed by the majority of the world population as their staple food [1]. As part of the meal, rice grains are consumed in the polished form in several countries and known as white rice. The outer layer of brown rice as known as bran, is a by-product in the rice milling industry. However, there is also pigmented rice grain available with reddish, purple or even blackish color. The red and black paddy varieties have about 38% more protein, about 18% more crude fiber and are richer in lysine, vitamin B1 and other minerals as compared to the conventional varieties. In addition to good quality protein, high fiber content and vitamin content, pigmented rice varieties deliver functional properties due to the antioxidant compounds they contain and have the ability to inhibit the formation of reactive cell-damaging free radicals. Anthocyanins as prime metabolites in the bran layers of the rice kernel have been reported as health-promoting functional food ingredients, which possessed anticancer, antioxidant activity, hypoglycemic and anti-inflammatory property [2]. Consequently, in these past years, the interest in pigmented rice (black and red) consumption has been rapidly increasing among the consumers due to their healthy functional food ingredients [3]. Keeping in view the several health benefits associated with anthocyanins, such as anti-inflammatory, antioxidative and anticancer effects, pigmented rice is considered a functional food and food ingredient [4]. In terms of food experience, the consumption of colored rice has increasingly become popular in many Asian and western countries where black rice is often mixed with non-pigmented rice before cooking to enhance the flavor and texture. Moreover, red rice is also used as a food-colorant in various products like bread, ice cream and liquor in several countries.
2. Pigmented Rice Varieties
Rice belongs basically to either japonica and indica subspecies. The indica of larger diversity has been divided into two subpopulations viz., indica and aus, whereas the japonica is subcategorized into the tropical japonica, temperate japonica and aromatic subpopulations. In addition to these five major rice subpopulations, a new group, rayada [5][6]. Within those, pigmented rice varieties are divergent with different qualitative and quantitative traits [7]. The obvious characteristic of this rice type is they are found to be mainly with red, black or purple pericarps. A large number of these varieties are being cultivated in various parts of the world as traditional landraces that in fact their nutritional qualities have been shown to be higher than that of the grain grown by conventional or the commercial varieties, vastly due to their more effective accumulation of nutraceuticals [8][9]. There are many cultivars of rice that contain colored pigments, and these pigments are generally localized in the pericarp or in the bran of the rice kernel that resulted in different colors of rice such as brown, red, purple and black. The dark purple color of black rice has been found to be due to the higher content of anthocyanins, which belong to the family of flavonoids and are located in the pericarp layers [5]. While the red pericarps are due to proanthocyanidins. Among the different classes of flavonoids present in pigmented rice, flavonols and flavan-3-ols are of great interest in the human diet. These flavonoids have received considerable attention in recent years due to their potential health benefits by acting as protective agents against cardiovascular disease and towards oxygen-free radical scavenging activity [10][11].
Black or purple rice, such as those belonging to the indica and japonica varieties, has a purple–black pericarp and pigmented leaves, as shown in Figure 1. The grain possesses pigments in the aleurone layer that is a mixture of anthocyanins. Rice of this type is grown massively in China, followed by Sri Lanka, Indonesia, India, Philippines, while Thailand ranked the ninth position for the world's cultivation volume [12]. Rice possessing red bran layer is called red rice or Raktasali; though the color in the rice grains is confined mainly to the bran layer, a tinge of red remains even after the milling process [13]. The pigments are naturally occurring compounds that belong to the family of flavonoids in which cyanidin, pelargonidin, peonidin, delphinidin, petunidin, and malvidin represent the most commonly occurring anthocyanin aglycons [14][15]. Pramai and Jiamyangyuen [16] pigmented Thai rice into red and black (purple) types based on their excellent sources of total phenolic, flavonoid and α-tocopherol contents and antioxidant capacities. With each type, these contents, as well as the intensity of pigmentation, may vary with the environment in which they are grown [17]. As mentioned, the local varieties of pigmented rice are usually more nutritious than those of the commercials [18][19][20]. Bran extract from the Northern Thai black rice variety (cv. Chiang Mai) was found to contain higher phenolic acid, flavonoids and anthocyanins (cyanidin 3-glucoside, peonidin 3-glucoside, cyanidin chloride) and was richer in antioxidant activities than conventional varieties [12]. In the screening tests for antioxidant activities, extracts from local pigmented rice grains such as Jumlalocal-1, Parnkhari 203, DZ78, LK1-3-6-12-1-1, and Elwee had higher antioxidative activity than did the non-pigmented variety [19]. The metabolites flavonoids with higher antioxidant potentials, namely 9-flavonoids, 9-hydroxycinnamic acid derivatives, 3-hydroxybenzoic acid derivatives and other glucosides specifically, proanthocyanidin trimer and procyanidin-B1 (dimer), were identified from bran extracts of the Indian colored rice cultivars included Zag, Kaw quder, Shel kew of the red-colored, Samarkand of black colored and Kaw kareed of light blackish colored [21]. The cyanidin and malvidin extracts from the bran of the local Korean pigmented rices cv. Heugjinjubyeo had been found to inhibit U937, human monocytic leukemia cells [22].

Figure 1. Examples of local Indian and Thai pigmented rice verities (Oryza sativa).
3. Phytochemicals and Antioxidant and Medicinal Values of Pigmented Rice Bran
As shown in Figure 2, rice bran is one of the most abundant co-products in the rice milling industry for animal feed, and its use as human food has been underutilized. Knowledge in phytochemistry, however, advises that this raw material is an excellent source of vitamins, minerals, essential fatty acids, dietary fiber and other sterols [23]; therefore, rice bran could be recognized as a valuable source for formulation of a diverse range of nutraceuticals and functional food products. Different varieties of rice have been found to affect differently in the human body due to their diverse inherent quality characteristics [22][24][25][26]. The ayurvedic practitioners have profound knowledge regarding the pharmacological properties of rice bran in curing different types of ailments [9][27]. In traditional Chinese medicine, consuming pigmented rice is known for nourishing Yin with the qualities of strengthening kidney function, treating anemia, promoting blood circulation, removing blood stasis, improving blood flow and detumescence, treating diabetes and ameliorating sight [28]. Hegde et al. [29] added several therapeutic benefits of pigmented rice bran, including its influence in the treatment of various ailments such as diarrhea, vomiting, fever, hemorrhage, chest pain, wounds and burns. Rice, due to its least allergic properties, is recommended for patients suffering from irritable bowel syndrome. The oryzanol in rice bran inhibit lipid peroxidation induced by UV light and thus could be used in cosmetics as sunscreen agents as well as a remedy for wrinkles and hyperpigmentation. The presence of ferulic acid and its esters in gamma-oryzanol has been found to stimulate hair growth, reducing atopic dermatitis [27]. Pigmented rice extracts have been revealed to effectively reduce inflammation and oxidative stress as well as atherosclerotic lesions [30].

Figure 2. Rice bran characteristics derived from the 30 s polishing process of (a) purple rice cv. Kum Doi Saket (KDSK) and (b) red rice cv. Yamuechaebia Morchor (YMCB CMU).
Besides being a major source of energy for humans, rice bran has higher levels of several phytochemicals that are having antioxidant activities and other health-promoting properties. Among these phytochemicals, vitamin E, including the four homologs (a, b, c and d) of tocopherols and tocotrienols and the γ-oryzanol. These components have an emerging potential in the formulation of functional foods and nutraceuticals. The physiologic effects associated with γ-oryzanol and other components present in rice bran included a reduction in atherosclerosis, accelerated excretion of fecal bile acids and inhibition of platelet aggregation [31]. The increasing interest in the exploration of effective and economical natural antioxidants as compared to synthetic antioxidants could be attributed to the adverse toxicity and carcinogenicity associated with synthetic antioxidants.
It has also been found that rice bran contains phenolic content higher than that of wheat bran [26]. These phenolic compounds and flavonoid contents found mostly in pigmented rice are having the potential antioxidant capacity, and these phytochemicals also act as metal ion chelators, free radical scavengers and reducing agents [28]. The scavenging of free radicals by the bioactive phytochemicals, like the phenolic compounds present in whole rice grains, may be a mechanism that ensures whole grains for their protective effects. Most of the phytochemicals in the whole rice grain are present in the bran fraction consisting of bran layers and the germ. Phytochemicals in whole grain rice are mainly found in hydrophilic, lipophilic and insoluble forms (Figure 3). The major lipophilic fractions of whole rice grains are tocopherols, tocotrienols and γ -oryzanol that are having potential health benefits. Whole rice grains also contain hydrophilic phenolic compounds such as tricin, caffeic acid, ferulic acid and methoxycinnamic acid that are having anti-carcinogenic properties [32]. Moreover, many epidemiological studies have shown that consumption of whole rice grains reduces the incidences of chronic diseases [33]. Rice grains contain unique free phenolic compounds and their glycosides in a significant amount as insoluble phenolic compounds that are mostly bound to polysaccharides in the cell wall. These compounds are concentrated mainly in the bran layers and are lost with the separation of seed coat during processing.
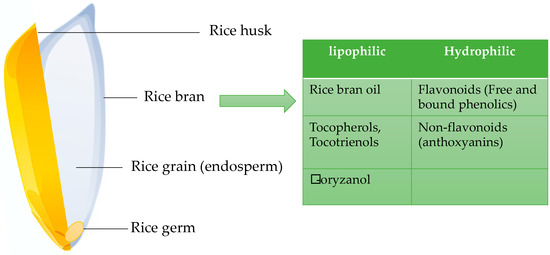
Figure 3. The phytochemical components of whole grain rice.
Even though whole rice grains have been strongly recommended by many governmental and health organizations for consumption purposes due to its health benefits, most of the phenolic compounds are also lost along with the processing of rice as bran. The great diversity of the phenolic compounds composed in the bran portion of brown rice cultivars implied their potential beneficial effects when consumed as whole rice. Researchers urged that antioxidant activity and phenolic content of black rice has been found to be higher than that of white rice [34]. Pigmented rice brans are highly valued in local markets, and greater interest has been shown in these rice cultivars recently due to the polyphenols in this rice that are having multiple biological activities. These phenolic compounds have been found to consist of anthocyanidins, ferulic acid and diferulates, anthocyanins and polymeric proanthocyanidins (condensed tannins). Phenolics act as reducing agents due to their ability to donate hydrogen atoms. Phenolics also act as singlet oxygen quenchers and free radical hydrogen donors, and because of these properties, they have protected the cell constituents against oxidative damage [35]. The antioxidant properties of phenolics have been reported in epidemiological studies as anti-carcinogenic and prevent cardiovascular and nerve diseases [35][36]. In addition, zinc and iron content in red rice has been reported to be 2–3 times higher than that of white rice [37]. Due to the availability of anthocyanins, acetylated procyanidin and other phenolics, rice bran also possesses significant free radical scavenging activity. Rice bran contains a unique complex of naturally occurring antioxidant compounds that are having professed health benefits as well as their ability to improve the storage stability of foods products having bran in their formulation. It has been reported that rice bran may contain even 100 different antioxidants, as new ones are being discovered. The most powerful among these are oryzanols, tocopherols and tocotrienols [38]. In the Indian pigmented rice variety, Gull zag, the superior antioxidant activities found in pigmented bran could be attributed to their greater amount of polar phenolic compounds such as thymol, quinicquinic-cafeic acid ester, tricafeoyl-hydroxyferulic acid and chlorogenic acid [39].
Rice bran has drawn the attention of chemists and pharmacologists in recent years due to the availability of some valuable vitamin E, including the four homologs (a, b, c and d) of tocopherols and tocotrienols and the c-oryzanol it contained. It has also been found that rice bran contains phenolic content higher than that of wheat bran [26]. A great deal of interest has been shown between the consumption of pigmented rice (with barn) and the improvement of human health due to the great antioxidant potency of phenolic compounds present in these rice varieties [40]. The health benefits of the flavonoids found in the pigmented rice barn are usually associated with two properties, including (i) inhibition of certain enzymes such as xanthine oxidase, aldose reductase and (ii) aldose reductase catalyzes the NADPH-dependent reduction of several sugar-derived carbonyl compounds into their respective sugar alcohols (e.g., sorbitol), that act in the development of diabetic complications such as retinopathy and neuropathy [40][41].
Pigmented rice varieties contain antioxidative compounds that are having the ability to inhibit the formation or to reduce the concentrations of reactive cell-damaging free radicals and thus have the potential to promote human health. These compounds include anthocyanins polymeric procyanidins; (glycosides)-cyanidin-3-O-β-D-glucoside and peonidin-3-O-β-glucoside anthocyanidins (aglycones)-cyanidin and malvidin; the phenolic compounds syringaldehyde, anisole, 4-hydroxycinnamic acid (p-coumaric), 4, 7-dihydroxyvanillic acid, protocatechuic acid methyl ester, vanillin, ferulic and sinapinic acids. These observations suggest that pigmented non-polished rice varieties may have beneficial effects on the human diet. These rice cultivars can also be employed by plant breeders in developing new hybrid rice varieties with higher antioxidant activities [19]. Pigmented rice varieties contain antioxidative compounds that are having the ability to inhibit the formation or to reduce the concentrations of reactive cell-damaging free radicals and thus have the potential to promote human health. These observations suggest that pigmented non-polished rice varieties may have beneficial effects on the human diet. Consuming pigmented rice is considered good for the treatment of fevers and ulcers; this also improves eyesight, voice improver, semen enhancer, diuretic, spermatophytic, refrigerant, cosmetic user, a tonic and antitoxic. Colored rice varieties have been reported to possess antioxidant properties [42] and considered more nutritious, being rich in Fe, Zn and minerals. These rice varieties have been found to reduce atherosclerotic plaque by 50% more than white rice in rabbits [43]. The clinical trials conducted in the USA have concluded that red rice yeast reduces cholesterol and total triglycerides, providing a novel food-based approach to lowering cholesterol [44]. It also stimulates protein secretion besides having radical scavenging effects [45]. Traditional pigmented and scented rice varieties have been revealed by scientists to possess a higher amount of Fe and Zn and helps in the bioavailability of iron [46]. The distribution of Fe and Zn in different grain layers is known to play a subsequentially role antioxidative capacity of rice grain [20].
4. The Anthocyanins in Rice Bran
Colored rice is considered enriched rice for taste and health benefits due to the presence of anthocyanins. The anthocyanins belong to a group of flavonoids and are water-soluble pigments. The six common anthocyanidins found in rice bran are cyanidin, delphinidin, peonidin, pelargonidin, petunidin and malvidin (Scheme 1). Anthocyanins are considered beneficial to health due to their higher antioxidant activity [14][38][47][48]. Cyanidin and peonidin present in black rice have been reported to suppress oxidative modification of human low-density lipoprotein and reduces the formation of nitric oxide. Cyanidin and malvidin have been revealed to mediate cytotoxicity by arresting the G2/M phase of the cell cycle and by induction of apoptosis. Anthocyanins present in red and black rice also leads to the reduction of atherosclerotic plaque formation [22][24][43].
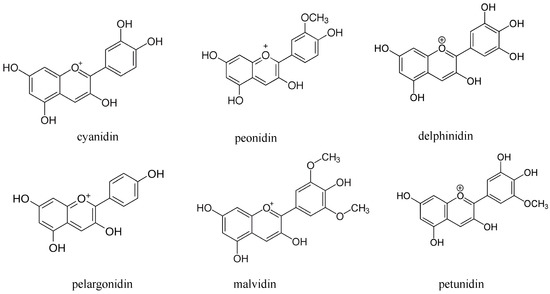
Scheme 1. The common anthocyanidins found in rice bran.
The important bioactive compounds present in rice bran are represented in Table 1.
Table 1. Bioactive compounds present in rice bran.
|
Flavonoid |
Phenol |
Hydroxycinnamic Acid Derivative |
Hydroxybenzoic Acid Derivatives |
Anthocyanin |
|
Epigallocatechin- 3-O-gallate |
Ellagic acid |
Ferulic acid hexose derivative |
p-hydroxybenzoic acid |
Cyanidin-3-O-rutinoside |
|
2-O-pentosyl-8-C-hexosyl-apigenin |
Phloretic acid |
p-Coumaroyl glucose |
Dihydrogallic acid derivative |
Pelargonidin-3-O-diglucoside |
|
Quercetin-3-O-(6-acetyl) glucoside. |
Thymol |
Caffeic acid |
Dicaffeoyl-protocatechuic acid diglucoside |
5- pyranopelargonidin- 3-O-glucoside |
|
(epi)-catechin |
(E)- Coniferaldehyde |
dicaffeoyl-protocatechuic acid diglucoside |
Dihydroxybenzoic acid-O-pentoside |
Pelargonidin-malonylrhamnoside |
|
Myrecitin |
Chlorogenic acid |
p-Coumaric acid |
Syringic acid |
Cyanidin-3-O-rutinoside |
|
Procyanidin B1 |
Thymol |
Dicaffeoylquinic acid |
Protocatechuic acid |
Pelargonidin-3-O-diglucoside |
|
Luteolin |
quinicquinic- caffeic acid ester |
Ferulic acid |
|
5- pyranopelargonidin- 3-O-glucoside |
|
Quercetin-3-O-rhamnoside |
Ellagic acid deoxyhexoside |
tricaffeoyl-hydroxyferulic acid |
|
|
|
Apigenin |
Phloretic acid |
5-O-feruloylquinic acid |
|
|
|
apigenin-6,8-di-C-glucoside |
|
|
|
|
|
Quercetin-3-O-rutinoside |
|
|
|
|
|
Apigenin-7-O-glucoside |
|
|
|
|
|
Quercetin-3-O-galactoside |
|
|
|
|
|
Quercetin pentosyl-pentoside |
|
|
|
|
5. Free and Bound Phenolic Compounds in Rice Bran
Phenolic acids are mainly present in the bran layers of rice existing as free, conjugated and bound forms; (i) free phenolics which may be readily extracted from rice bran and (ii) bound phenolics which are covalently bound to cell wall components such as cellulose, hemicellulose, lignin, pectin and structural proteins. The bound phenolics present in rice bran amounted to more than 70% of the total phenolic and are having a higher antioxidant capacity than free phenolics. These bound phenolics are not easily extracted in aqueous or organic solvents, and to improve their extractability, chemical degradation or disruption of plant cell wall matrices has been commonly employed by acid or base hydrolysis [49]. However, those treatments are not desired to be practiced at a commercial scale because the degradation often results in increasing viscosity due to fiber dissolution and thus inhibiting the extraction of phenolic compounds [25]. The free phenolic compounds after absorption in the human body can take action, either on-site or at remote sites, thus prevents the incidence of colon cancer and other chronic diseases [50]. In wild rice, some phenolic acids, such as diferulic acid and disinapic acid, are even dimerized and esterified to the cell wall. Ferulic, p-coumaric, syringic, and isoferulic acids are present as bound forms in white, red and black rice grains. The content of phenolic acids in brown rice is significantly higher than in milled rice. The distribution of phenolic acids varies in botanical parts of the rice kernel. Ferulic acid is predominant in the bran, whereas vanillic and p-coumaric acids are concentrated in the husk. Phenolic acids display positive effects on several human chronic diseases, such as cardiovascular, cancer, obesity, diabetes. Their content in rice may be related to grain color, size and weight and controlled by genetic factors [51]. Rice bran was identified as a rich source of several phenolic compounds, including free and bound phenolic acids, such as ferulic and p-coumaric acids, tannins, lignins and flavonoids. Flavonoids are water-soluble and are among the most common group of phenolic compounds that are powerful antioxidants, thus providing various human health benefits. Literature has revealed that the bran of red and black rice cultivars exhibit higher phenolic contents and antioxidant activity than bran of non-pigmented rice varieties [50]. The majority of phenolic compounds in rice bran are bound covalently to cell wall components, and some of these are hydrolyzed from their attached macromolecule by intestinal enzymes. However, some phenolic compounds reach the colon in intact form, where they are released by colonic microflora [18].
The diversity in phenolic and flavonoid compounds results in higher demand for their identification and quantification by employing different analytical methods [47]. Different aspects of the HPLC method have been adapted to improve the separation and quantification of desired compounds, including the stationary phase (column), mobile phase composition, gradient program and column temperature. The aim of using different detectors may be to quantify the compounds from multiple phenolic groups. A sensitive, accurate and specific method coupling high-performance liquid chromatography–diode array detection (HPLC–DAD) coupled with electrospray ionization tandem mass spectrometry (ESI-MS/MS) has become one of the most popular methods applied for identification and quantification of phenolic acids, flavonoid glycosides and aglycones in the extracts of rice and rice bran [52]. Flavonoids in rice bran included a diverse group of polyphenolic secondary metabolites that possess free radical scavenging properties and played an important role in regulating a number of biological functions such as antiviral, antibacterial, anti-inflammatory, antithrombotic, anti-allergic [39].
6. Rice Bran Oil
Rice bran oil is extracted from the hard outer brown layer of dehusked rice. It is recommended due to its higher smoking point (232 °C) and mild flavor; thereby, it is considered desirable for high-temperature cooking processes [53]. As it is light and quite versatile, it can even be used in cookies and cakes with a mild nutty flavor. In cooking, the oil is used for sautéing, grilling, marinades and salad dressings. Rice bran oil is less viscous, and it does not stick to food, which leads to less absorbance of oil in food products cooked at high temperatures; thus, it is beneficial in reducing overall calories. It maintains its nutritive quality even at high temperatures. One of the major components in rice bran oil is the antioxidant γ-oryzanol, at around 2% of crude oil content [54]. Furthermore, significant is the relatively high fractions of tocopherol and tocotrienols, together as vitamin E. Rice bran oil has been recommended by The World Health Organization (WHO) as the best substitute for improving serum cholesterol levels [55]. Results of an animal study indicated a 42% decrease in total cholesterol with a 62% drop in LDL cholesterol when researchers supplemented test subjects' diets with fractionated vitamin E obtained from rice bran oil [56]. Rice bran oil is called as heart-friendly oil due to its ability to decrease the absorption of cholesterol and enhances its elimination. Rice bran oil contains substances that might decrease calcium absorption, which in turn helps to reduce the formation of certain types of kidney stones [57]. As per literature, γ-oryzanol, present in rice bran oil, has been found to provide relief from hot flashes in about 90% of women after taking a supplement of the purified concentrate for four to six weeks, which may be associated with its phytoestrogen property [58].
Rice bran oil contains antioxidants in excessive amounts in comparison to other cooking oils. This essentially results in health benefits like squalene softens the skin as it is a natural moisturizer. This effectively helps delay wrinkle formation and protects the skin from sun damage, and maintains a healthy skin tone. Enhances the immune system due to its high antioxidant content; it reduces the free radicals that harm the immune system, thereby protecting the body from disease. Tocopherol and tocotrienols (vitamin E) in bran oil are powerful antioxidants that eliminate cancer-causing free radicals, thereby reducing cancer risk. Vitamin E in rice bran oil helps improve neurological functioning and balances the endocrine hormones [59].
Solvent extraction by hexane is the most widely used method for extracting rice bran oil. The conventional methods of solvent extraction are the complex method of the oil extraction process and are energy-intensive and raise the costs for the solvent extraction of oil significantly [60]. Extraction by means of ultrasonication techniques has recently become popular as a novel alternative to the traditional method of solvent extraction by offering numerous advantages such as shorter treatment times, simpler sample preparation and higher efficiency. Hence extraction by means of ultrasonication using hexane as a solvent was the principal technique investigated in this research. Among several innovative methods to be used in the solvent extraction process, two methods have significant success in the extraction process, that are microwaves and ultrasound. Microwave-assisted extraction (MAE) is a thermal process, while ultrasound-assisted solvent extraction (UASE) is a more mechanical process. Even though MAE has been proven to extract several valuable phytochemicals [61][62][63], the thermal nature of the method makes it unsuitable for the extraction of polyunsaturated fatty acids (PUFAs) due to their high susceptibility to oxidation and degradation. Ultrasonication, on the other hand, has been successfully used for the extraction of oils from different biomaterials, including rice bran, to varying degrees of success [64]. Ultrasound-assisted solvent extraction with hexane as a solvent could be used to extract high-quality rice bran oil with greater efficiency than conventional methods. Ultrasound has evolved from emerging technology within the last ten years and has developed into a fully commercial processing technology. High reliability and scalability, as well as low maintenance costs and high energy efficiency, make ultrasound a promising contender for established oil extraction equipment. Ultrasonication improves the extraction of oil from the raw bran oil. In comparison to the traditional Soxhlet method, ultrasonication results in improved oil extraction from rice bran by breaking the cell structure, which results in the release of more lipids stored inside the cells by the application of outside pressure by ultrasonication [65].
7. Scope in the Production of Medicinal Food with Nutraceutical Importance
The application and incorporation of bran into food products for the preparation of functional foods is limited due to its coarse texture and low concentration of the bioactive compounds. The properties of bran can be improved by fermentation technology in which many biochemical changes take place and thus result in an increase in nutritive and anti-nutritive components of bran that, in turn, affects its properties such as bioactivity and digestibility [66]. Improvement of bioavailability of rice bran phenolics in terms of release of insoluble bound phenolics could be achieved by means of an aqueous phase, chemical, enzymatic and fermentation methods [67][68][69]. Insoluble bound phenolics in bran have been found to be linked with arabinoxylans that are present in the cell wall by means of ester linkages [70]. Rice bran has drawn the attention of chemists and pharmacologists in recent years due to the availability of its valuable functional compounds. As a rich source of dietary fiber, rice bran has great potential in the development of functional foods, including bakery products. The development of nutraceuticals using fiber of rice bran has been found to control both type I and type II diabetes mellitus [71]. The peptides extracted from rice bran had been found to have an essential role in inhibiting obesity [42]. Supplementation of rice bran in functional foods serves in reduces cholesterol, improving cardiovascular health and anti-carcinogenic property [24]. Stabilized rice contains β-sitosterols, which play an important role in inhibition and apoptosis of cancer cells in the breast [72]. Rice bran contains essential amino acids, including tryptophan, histidine, methionine, cysteine and arginine, which could be utilized for the enrichment of functional foods. Supplementation of rice bran had been found to enhance the growth and colonization of Lactobacillus rhamnosus, thereby improves the health of the gut by preventing human rotavirus infection [73]. Supplements based on rice bran have been used by bodybuilders and athletes as ergogenic supplements and also reduce bone loss due to osteoporosis in postmenopausal women's [74]. The micronutrients present in rice bran like magnesium, calcium, phosphorous, manganese, and B-vitamin find their application in emerging nutraceutical development industries. The flavonoids anthocyanins that are present in bran of pigmented rice have been considered as an essential nutraceutical due to its antioxidant and pharmacological properties [75]. Anthocyanin in bran had been reported to be essential in enhancing protein homeostasis and neuroprotection [76]. Rice bran has been reported to decrease the chances of formation of kidney stones due to its ability to reduce the absorption of calcium. The rice bran has been found to possess antidiabetic and antidyslipidemic activities in several animal model trials [77]. It has also been validated to prevent oxidative stress in rat and mouse models [78].
High-value-adding components from plants are susceptible to external factors like light, heat or pH changes. Encapsulation is a good method to protect these unstable compounds and to preserve them against environmental stresses. Encapsulation is a process where the compounds of interest are enveloped by one or a mixture of polymers referred to as matrix materials. As a result, it protects compounds from relatively rapid degradation and helps to control the release of these compounds. Rice bran dietary fiber and protein has been used as carrier agents for the encapsulations for food and drug applications [79][80][81]. Besides the dietary fiber also illustrates several functional properties like water holding capacity (WHC), oil holding capacity (OHC), thickening and emulsifying abilities that may also be used as a food additive [82].
This entry is adapted from the peer-reviewed paper 10.3390/agronomy10111817
References
- Tyagi, A.K.; Khurana, J.P.; Khurana, P.; Raghuvanshi, S.; Gaur, A.; Kapur, A.; Gupta, V.; Kumar, D.; Ravi, V.; Vij, S.; et al. Structural and functional analysis of rice genome. J. Genet. 2004, 83, 79–99, doi:10.1007/Bf02715832.
- Min, S.W.; Ryu, S.N.; Kim, D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010, 10, 959–966, doi:10.1016/j.intimp.2010.05.009.
- Pandey, S.; Asha, M.R.; Jayadep, A. Changes in physical, cooking, textural properties and crystallinity upon iron fortification of red rice (Jyothi). J. Food Sci. Technol. 2016, 53, 1014–1024, doi:10.1007/s13197-015-2130-7.
- Shipp, J.; Abdel-Aal, E.-S. Food Applications and Physiological Effects of Anthocyanins as Functional Food Ingredients. Open Food Sci. J. 2010, 4, doi:10.2174/1874256401004010007.
- Mbanjo, E.G.N.; Jones, H.; Caguiat, X.G.I.; Carandang, S.; Ignacio, J.C.; Ferrer, M.C.; Boyd, L.A.; Kretzschmar, T. Exploring the genetic diversity within traditional Philippine pigmented Rice. Rice 2019, 12, 27, doi:10.1186/s12284-019-0281-2.
- Wang, C.H.; Zheng, X.M.; Xu, Q.; Yuan, X.P.; Huang, L.; Zhou, H.F.; Wei, X.H.; Ge, S. Genetic diversity and classification of Oryza sativa with emphasis on Chinese rice germplasm. Heredity 2014, 112, 489–496, doi:10.1038/hdy.2013.130.
- Embate, M.V.G.; Calayugan, M.I.C.; Gentallan, R.P.; Sta Cruz, P.C.; Hernandez, J.E.; Borromeo, T.H. Genetic diversity of selected pigmented traditional rice (Oryza sativa L.) varieties from Mindanao, Philippines using agromorphological traits and simple sequence repeats markers. J. Crop. Sci. Biotechnol. 2020, 10.1007/s12892-020-00075-0, doi:10.1007/s12892-020-00075-0.
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229, doi:10.3389/fgene.2020.00229.
- Bhat, F.; Riar, C. Health benefits of traditional rice varieties of temperate regions. Med. Aromat. Plants 2015, 4, 198.
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Coisson, J.D.; Arlorio, M. Characterisation of polymeric skin and seed proanthocyanidins during ripening in six Vitis vinifera L. cv. Food Chem. 2011, 127, 180–187, doi:10.1016/j.foodchem.2010.12.141.
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081, doi:10.1111/ijfs.13378.
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 331–338, doi:10.1590/1678-457X.6573.
- Yamuangmorn, S.; Dell, B.; Rerkasem, B.; Prom‐u‐thai, C. Applying nitrogen fertilizer increased anthocyanin in vegetative shoots but not in grain of purple rice genotypes. J. Sci. Food Agric. 2018, 98, 4527–4532.
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697, doi:10.1016/j.foodres.2011.07.037.
- Sun, D.; Huang, S.; Cai, S.; Cao, J.; Han, P. Digestion property and synergistic effect on biological activity of purple rice (Oryza sativa L.) anthocyanins subjected to a simulated gastrointestinal digestion in vitro. Food Res. Int. 2015, 78, 114–123, doi:10.1016/j.foodres.2015.10.029.
- Pramai, P.; Jiamyangyuen, S. Chemometric classification of pigmented rice varieties based on antioxidative properties in relation to color. Songklanakarin J. Sci. Technol. 2016, 38.
- Rerkasem, B.; Jumrus, S.; Yimyam, N.; Prom-u-Thai, C. Variation of grain nutritional quality among Thai purple rice genotypes grown at two different altitudes. Science 2015, 41, 377.
- Muntana, N.; Prasong, S. Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pak. J. Biol. Sci. 2010, 13, 170–174, doi:10.3923/pjbs.2010.170.174.
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Koh, H.J.; Kozukue, N.; Friedman, M. Antioxidative activities of bran extracts from twenty one pigmented rice cultivars. Food Chem. 2006, 94, 613–620, doi:10.1016/j.foodchem.2004.12.010.
- Prom-U-Thai, C.; Jamrus, S.; Jaksomsak, P.; Rouached, H.; Rerkasem, B. Iron, Zinc and Total Antioxidant Capacity in Different Layers of Rice Grain among Different Varieties. Int. J. Agric. Biol. 2016, 18, 1131–1136.
- Bhat, F.M.; Riar, C.S. Extraction, identification and assessment of antioxidative compounds of bran extracts of traditional rice cultivars: An analytical approach. Food Chem. 2017, 237, 264–274, doi:10.1016/j.foodchem.2017.05.113.
- Hyun, J.W.; Chung, H.S. Cyanidin and Malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G(2)/M phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217, doi:10.1021/jf030370h.
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30, doi:10.1016/j.bcdf.2015.06.002.
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J. Agric. Food Chem. 2003, 51, 5271–5277, doi:10.1021/jf034466n.
- Kim, S.-M.; Lim, S.-T. Enhanced antioxidant activity of rice bran extract by carbohydrase treatment. J. Cereal Sci. 2016, 68, 116–121, doi:10.1016/j.jcs.2016.01.006.
- Lai, P.; Li, K.Y.; Lu, S.; Chen, H.H. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chem. 2009, 117, 538–544, doi:10.1016/j.foodchem.2009.04.031.
- Burlando, B.; Cornara, L. Therapeutic properties of rice constituents and derivatives (Oryza sativa L.): A review update. Trends Food Sci. Technol. 2014, 40, 82–98, doi:10.1016/j.tifs.2014.08.002.
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic Compounds and Bioactivities of Pigmented Rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306, doi:10.1080/10408398.2010.529624.
- Hegde, S.; Yenagi, N.; Kasturiba, B. Indigenous knowledge of the traditional and qualified ayurveda practitioners on the nutritional significance and use of red rice in medications. Indian J. Tradit. Knowl. 2013, 12, 506–511.
- Saenkod, C. Anti-oxidative biochemical properties of extracts from some Chinese and Thai rice varieties. Afr. J. Food Sci. 2013, 7, 300–305, doi:10.5897/AJFS2013.1010.
- Bapputty, R.; Krishnakantha, T.P.; Lokesh, B. Lowering of platelet aggregation and serum eicosanoid levels in rats fed with a diet containing coconut oil blends with rice bran oil or sesame oil. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 151–160, doi:10.1016/j.plefa.2010.06.004.
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170.
- Seal, C.J.; Brownlee, I.A. Whole-grain foods and chronic disease: Evidence from epidemiological and intervention studies. Proc. Nutr. Soc. 2015, 74, 313–319.
- Kushwaha, U.K.S. Black rice. In Black Rice; Springer, Berlin: 2016; pp. 21–47.
- Sommano, S. Effect of Food Processing on Bioactive Compounds. In Advances in Food Science and Nutrition, 2nd ed.; P.M., V, Iturriaga, L.B., Ribotta, P.D., Eds.; Scrivener Publishing LLC, Beverly, MA: 2013; 10.1002/9781118865606.ch11pp. 361-390.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897, doi:10.1016/j.jff.2015.06.018.
- Saxena, A. Save the Red Rice: A Unique Gift of Nature. Int. J. Curr. Res. Biosci. Plant Biol. 2014, 1, 32–34.
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ‐oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104.
- Bhat, F.; Riar, C. Extraction solvent concentration affecting the anthocyanins and other phytochemicals profile and antioxidant properties of bran extracts of pigmented rice cultivars. Sci. Iran. 2018, 25, doi:10.24200/SCI.2018.20616.
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625.
- Narayanaswamy, R.; Wai, L.K.; Esa, N.M. Molecular docking analysis of phytic acid and 4-hydroxyisoleucine as cyclooxygenase-2, microsomal prostaglandin E synthase-2, tyrosinase, human neutrophil elastase, matrix metalloproteinase-2 and-9, xanthine oxidase, squalene synthase, nitric oxide synthase, human aldose reductase, and lipoxygenase inhibitors. Pharmacogn. Mag. 2017, 13, S512.
- Zhang, X.; Shen, Y.; Prinyawiwatkul, W.; King, J.M.; Xu, Z. Comparison of the activities of hydrophilic anthocyanins and lipophilic tocols in black rice bran against lipid oxidation. Food Chem. 2013, 141, 111–116, doi:10.1016/j.foodchem.2013.03.034.
- Ling, W.H.; Cheng, Q.X.; Ma, J.; Wang, T. Red and black rice decrease atherosclerotic plaque formation and increase antioxidant status in rabbits. J. Nutr. 2001, 131, 1421–1426, doi:10.1093/jn/131.5.1421.
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236, doi:10.1093/ajcn/69.2.231.
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, I.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529, doi:10.1021/jf025841z.
- Chaudhary, R.C.; Tran, D.; Duffy, R. Speciality rices of the world: Breeding, production and marketing. Food and Agriculture Organization (FAO); Science publisher, Rome, Italy: 2001.
- Sommano, S.; Caffin, N.; Kerven, G. Screening for Antioxidant Activity, Phenolic Content, and Flavonoids from Australian Native Food Plants. Int. J. Food Prop. 2013, 16, 1394–1406, doi:10.1080/10942912.2011.580485.
- Sommano, S.; Caffin, N.; McDonald, J.; Cocksedge, R. Food safety and standard of Australian native plants. Qual. Assur. Saf. Crop. Foods 2011, 3, 176–184, doi:10.1111/j.1757-837X.2011.00109.x.
- Sommano, S.; Kumpoun, W.; Yusuf, N.A. Subcellular extraction and enzyme characterisation of polyphenol oxidase and peroxidase in Cinnamon myrtle. Acta Physiol. Plant. 2016, 39, 36, doi:10.1007/s11738-016-2339-8.
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501, doi:10.1016/j.foodchem.2006.02.006.
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218, doi:10.1016/j.jcs.2014.01.004.
- Chen, L.; Qi, J.; Chang, Y.-x.; Zhu, D.; Yu, B. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC–DAD–ESI-MS/MS. J. Pharm. Biomed. Anal. 2009, 50, 127–137, doi:10.1016/j.jpba.2009.03.039.
- Zhuang, X.; Yin, T.; Han, W.; Zhang, X. Chapter 10—Nutritional Ingredients and Active Compositions of Defatted Rice Bran. In Rice Bran and Rice Bran Oil; Cheong, L.-Z., Xu, X., Eds.; AOCS Press, United Kingdom: 2019; pp. 247–270.
- Dunford, N.T. Chapter 1—Chemistry of Rice Bran Oil. In Rice Bran and Rice Bran Oil; Cheong, L.-Z., Xu, X., Eds.; AOCS Press, United Kingdom: 2019; pp. 1–18.
- Wang, Y. Chapter 6—Applications of Rice Bran Oil. In Rice Bran and Rice Bran Oil, Cheong, L.-Z., Xu, X., Eds.; AOCS Press, United Kingdom: 2019; pp. 159–168.
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. 2005, 43, 747–753, doi:10.1016/j.fct.2005.01.015.
- Ohkawa, T.; Ebisuno, S.; Kitagawa, M.; Morimoto, S.; Miyazaki, Y.; Yasukawa, S. Rice bran treatment for patients with hypercalciuric stones: Experimental and clinical studies. J. Urol. 1984, 132, 1140–1145, doi:10.1016/s0022-5347(17)50065-8.
- Philp, H.A. Hot flashes-a review of the literature on alternative and complementary treatment approaches. Altern. Med. Rev. 2003, 8, 284–302.
- Nayik, G.; Majid, I.; Gull, A.; Muzaffar, K. Rice bran oil, the Future Edible Oil of India: A mini Review. J. Rice Res. 2015, doi:10.4172/2375-4338.1000151.
- Hammond, E.G.; Johnson, L.A.; Su, C.; Wang, T.; White, P.J. Soybean Oil. In Bailey's Industrial Oil and Fat Products; Wiley, New York, 2015.
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango Peel Pectin by Microwave-Assisted Extraction and Its Use as Fat Replacement in Dried Chinese Sausage. Foods 2020, 9, 450.
- Sommano, S.R.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665.
- Sommano, S.; Kerdtongmee, P.; Chompoo, M.; Nisoa, M. Fabrication and characteristics of phase control microwave power for jasmine volatile oil extraction. J. Essent. Oil Res. 2015, 27, 316–323, doi:10.1080/10412905.2015.1023904.
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochemistry 2008, 15, 898–902, doi:10.1016/j.ultsonch.2007.10.009.
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Effects of ultrasonication and thermal cooking pretreatments on the extractability and quality of cold press extracted rice bran oil. J. Food Process. Eng. 2019, 42, e12975, doi:10.1111/jfpe.12975.
- Prabhu, A. Effect of yeast fermentation on nutraceutical and antioxidant properties of rice bran. 2014, 3, 59–65.
- Abd Rashid, N.Y.; Abd Razak, D.; Jamaluddin, A.; Sharifudin, S.; Long, K. Bioactive compounds and antioxidant activity of rice bran fermented with lactic acid bacteria. Malays. J. Microbiol. 2015, 11, 156–162, doi:10.21161/mjm.12714.
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Zhang, Y.; Wei, Z.; Xiao, J.; et al. A Comparison of the Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds from Rice Bran and Its Dietary Fibres. Molecules 2018, 23, 202, doi:10.3390/molecules23010202.
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473, doi:10.1016/j.foodchem.2005.01.032.
- Kroon, P.A.; Garcia-Conesa, M.T.; Fillingham, I.J.; Hazlewood, G.P.; Williamson, G. Release of ferulic acid dehydrodimers from plant cell walls by feruloyl esterases. J. Sci. Food Agric. 1999, 79, 428–434, doi:10.1002/(SICI)1097-0010(19990301)79:3<428::AID-JSFA275>3.0.CO;2-J.
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 13, 175–187, doi:10.1016/s0955-2863(01)00211-x.
- Awad, A.B.; Downie, A.C.; Fink, C.S. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 2000, 5, 541–545, doi:10.3892/ijmm.5.5.541.
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function and innate immunity. Sci. Rep. 2015, 5, 15004, doi:10.1038/srep15004.
- Colona, H. The Effects of Oryzanol on Bone Mineral Density in Ovariectomized, Retired Breeder Rats. LSU Master's These, Louisiana State Univerity, Louisian, 2002.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933, doi:10.1016/S0031-9422(03)00438-2.
- Bhat, F.M.; Riar, C.S. Characterizing the pigmented traditional rice cultivars grown in temperate regions of Kashmir (India) for free and bound phenolics compounds and in vitro antioxidant properties. J. Cereal Sci. 2017, 76, 253–262, doi:10.1016/j.jcs.2017.06.018.
- Boonloh, K.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U.; Thawornchinsombut, S.; Pannangpetch, P. Rice Bran Protein Hydrolysates Improve Insulin Resistance and Decrease Pro-inflammatory Cytokine Gene Expression in Rats Fed a High Carbohydrate-High Fat Diet. Nutrients 2015, 7, 6313–6329, doi:10.3390/nu7085292.
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472, doi:10.1016/j.nut.2013.09.016.
- Jiang, G.; Ameer, K.; Eun, J.-B. Encapsulation of hot air-dried asian pear powders using rice bran dietary fiber. Food Biosci. 2020, 38, 100742, doi:10.1016/j.fbio.2020.100742.
- Abulencia, A.B.; Vidallon, M.L.P.; Almeda, R.A.; Salamanez, K.C.; Rodriguez, E.B. Rice bran phospholipid-based nanovesicles for enhanced oral and topical delivery of capsaicinoids. J. Drug Deliv. Sci. Technol. 2020, 60, 102005, doi:10.1016/j.jddst.2020.102005.
- Hasanvand, E.; Rafe, A. Development of vanillin/β-cyclodexterin inclusion microcapsules using flax seed gum-rice bran protein complex coacervates. Int. J. Biol. Macromol. 2019, 131, 60–66, doi:10.1016/j.ijbiomac.2019.03.066.
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351, doi:10.1016/j.lwt.2016.09.012.
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473, doi:10.1016/j.foodchem.2005.01.032.
- Kroon, P.A.; Garcia-Conesa, M.T.; Fillingham, I.J.; Hazlewood, G.P.; Williamson, G. Release of ferulic acid dehydrodimers from plant cell walls by feruloyl esterases. J. Sci. Food Agric. 1999, 79, 428–434, doi:10.1002/(SICI)1097-0010(19990301)79:3<428::AID-JSFA275>3.0.CO;2-J.
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 13, 175–187, doi:10.1016/s0955-2863(01)00211-x.
- Awad, A.B.; Downie, A.C.; Fink, C.S. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 2000, 5, 541–545, doi:10.3892/ijmm.5.5.541.
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function and innate immunity. Sci. Rep. 2015, 5, 15004, doi:10.1038/srep15004.
- Colona, H. The Effects of Oryzanol on Bone Mineral Density in Ovariectomized, Retired Breeder Rats. LSU Master's These, Louisiana State Univerity, Louisian, 2002.
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933, doi:10.1016/S0031-9422(03)00438-2.
- Bhat, F.M.; Riar, C.S. Characterizing the pigmented traditional rice cultivars grown in temperate regions of Kashmir (India) for free and bound phenolics compounds and in vitro antioxidant properties. J. Cereal Sci. 2017, 76, 253–262, doi:10.1016/j.jcs.2017.06.018.
- Boonloh, K.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U.; Thawornchinsombut, S.; Pannangpetch, P. Rice Bran Protein Hydrolysates Improve Insulin Resistance and Decrease Pro-inflammatory Cytokine Gene Expression in Rats Fed a High Carbohydrate-High Fat Diet. Nutrients 2015, 7, 6313–6329, doi:10.3390/nu7085292.
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472, doi:10.1016/j.nut.2013.09.016.
- Jiang, G.; Ameer, K.; Eun, J.-B. Encapsulation of hot air-dried asian pear powders using rice bran dietary fiber. Food Biosci. 2020, 38, 100742, doi:10.1016/j.fbio.2020.100742.
- Abulencia, A.B.; Vidallon, M.L.P.; Almeda, R.A.; Salamanez, K.C.; Rodriguez, E.B. Rice bran phospholipid-based nanovesicles for enhanced oral and topical delivery of capsaicinoids. J. Drug Deliv. Sci. Technol. 2020, 60, 102005, doi:10.1016/j.jddst.2020.102005.
- Hasanvand, E.; Rafe, A. Development of vanillin/β-cyclodexterin inclusion microcapsules using flax seed gum-rice bran protein complex coacervates. Int. J. Biol. Macromol. 2019, 131, 60–66, doi:10.1016/j.ijbiomac.2019.03.066.
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351, doi:10.1016/j.lwt.2016.09.012.
