Stem cell encapsulation is a technique that utilizes various biomaterials for the creation of a semi-permeable membrane that encases the stem cells. Stem cell encapsulation can be accomplished by employing a great variety of natural and/or synthetic hydrogels, and offers many benefits in regenerative medicine, including protection from host’s immune system and mechanical stress, improved cell viability, proliferation and differentiation, cryopreservation and controlled and continuous delivery of the stem cell secreted therapeutic agents. In this review, we report and discuss almost all natural and synthetic hydrogels used in stem cell encapsulation, along with the benefits that these materials, alone or in combinations, could offer to cell therapy through a functional cell encapsulation.
- stem cells
- encapsulation
- biomaterials
1. Introduction
Over the last few decades, cell-based therapies have been a novel therapeutic approach for several diseases. Various types of stem cells have been used in experimental models and clinical trials for the regeneration and functional restoration of specific damaged tissues and organs, and they have been proposed to have a central role in regenerative medicine [1][2]. These cells are characterized by their capacity for self-renewal and differentiation into specific cell types of all tissues and organ systems of the body, according to the microenvironmental conditions, preserving the damage repair ability in the host [3][4]. The transplantation of exogenous stem cells for the induction of tissue regeneration could be autologous and/or allogeneic (non-autologous) [2][5]. In cases of allogeneic stem cell administration, graft-versus-host disease is expected to occur and appropriate measures must be taken, as stem cells express the major histocompatibility complex (MHC) receptor and secrete soluble mediators for inviting immune cells and enabling this type of reaction [6][7]. For avoiding the consequences of host-versus-transplant reactions, cell-based therapeutic approaches, with the support of engineering technologies, have been developing new techniques that combine principles of material science and engineering with stem cell biology [8]. Cell immobilization techniques have been developed during the last few decades to provide several advantages, such as structural support and a controlled environment, to cells [9]. These techniques can be divided into two major categories, the entrapment of cells into scaffolds (cell-laden scaffolds) and the encapsulation of cells into hydrogels. Scaffolding technology includes a great variety of materials (including hydrogels) aiming to provide appropriate vehicles for cell seeding, while encapsulation is oriented towards micro-tuning specific hydrogels, creating cell-incorporated, semi-permeable “capsules” [10].
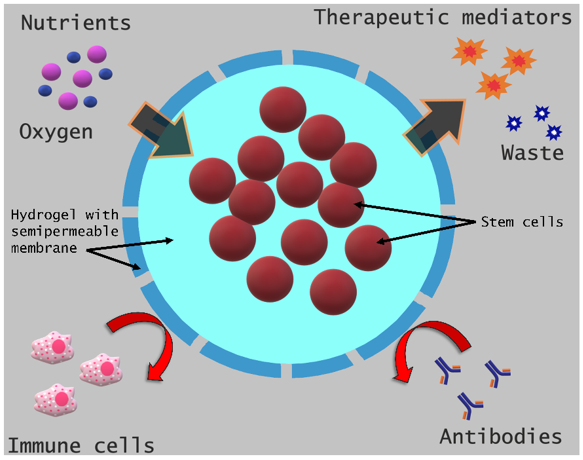
Encapsulation provides a semi-permeable barrier surrounding the cell, which, on the one hand, impedes the recognition of the implanted cell by the host’s immune system and its subsequent elimination and, on the other hand, allows the encapsulated cell to have access to nutrients and to maintain its functional potential, mainly through the secretion of soluble mediators that help in repairing the target tissue (Figure 1) [11].
Figure 1. Schematic of stem cell encapsulation and its benefits.
Since 1964, when Chang first presented cell encapsulation technology [12], a large number of cell encapsulation biomaterials have been used and proposed for a variety of diseases and pathologies [8][13][14]. The physicochemical properties of biomaterials appear to play an important role, thus dictating which ones should be utilized in specific applications in order to achieve optimized results in regenerative medicine interventions (Table 1).
Table 1. Natural and synthetic encapsulation biomaterials utilized in various medical applications.
|
Application |
Biomaterial |
|
Angiogenesis |
Gelan gum-HA [15], Alginate-Gelatin [16], PEG [17], HA-MAP [18] |
|
Bone tissue engineering |
Alginate [19], GelMA [20], Alginate-g-PNIPAAm [21], Collagen-Fibrin [22] |
|
Breast cancer |
Alginate [23] |
|
Cartilage tissue engineering |
CS-MA-PEGDA [24], CH-GP-HEC [25], PEG-PNIPAAm [26], Gelatin-HA [27] |
|
Cartilage/bone tissue engineering |
|
|
Diabetes |
PPS-b-PDMA-b-PNIPAAm [30] |
|
Foreign body response |
Alginate [31] |
|
Ischemic stroke |
CS-bFGF [32] |
|
Ischemic tissue engineering |
PNIPAAm-PHEMA-AOLA-PEGMA-PFO [33] |
|
Muscle tissue engineering |
Fibrin [34] |
|
Myocardial infarction |
PNIPAAm [35], PNIPAAm-PPAA-PHEMA-OTMC-PEGMA [36], PNIPAAm-PAA [37], PEG-fibrinogen [38], PNIPAAm-SWCNTs [39], HA-MAP [40] |
|
Neural and visual repairment |
HA and Methylecelluse [41] |
|
Sensorineural hearing loss |
Alginate (Ultra high viscous) [42] |
|
Spine injury |
Gelan gum [43], HA [44], PHEMA-APMA-PAMAM [45], L-PDMA-PNIPAAm [46], Alginate-Collagen [47], CS-MA [48] |
|
Wound healing (skin) |
|
|
Wound healing (diabetes) |
Poloxamer 407 [51] |
|
Wound healing (corneal) |
Alginate [52] |
|
Wound Healing (mucosal) |
PEG-4MAL-IFN-γ [53] |
AOLA: acrylate-oligolactide; APMA: N-(3-Aminopropyl)methacrylamide; b: block; bFGF: basic fibroblast growth factor; CH: chitosan; CS: chondroitin sulfate; g: graft; GelMA: gelatin methacrylate; GP: glycerophosphate; HA: hyaluronic acid; HEC: hydroxyethyl cellulose; IFN-γ: interferon gamma; L: laponite; MA: methacrylate; MAP: mussel adhesive protein; OTMC: oligo(trimethylene carbonate); PAMAM: polyamidoamine; PDMA: poly(dimethylacrylamide); PEG: poly(ethylene glycol); PEGDA: poly(ethylene glycol) diacrylate; PEGMA: methacrylate-poly(ethylene glycol); PFO: perfluorooctane; PHEMA: poly(2-hydroxyethyl methacrylate); PNIPAAm: poly(N-isopropylacrylamide); PPAA: polypropylacrylic acid; PPS: poly[(propylene sulfide; SAP: sodium ascorbyl phosphate; SWCNTs: single-wall carbon nanotubes; 4MAL: four-arm maleimide.
To date, several types of cells and stem cells (Table 2) have been used in encapsulation technology in various diseases; it is understood that different materials and techniques need to be developed in order to ensure the benefits of encapsulation in any case [14].
Table 2. Natural and synthetic biomaterials utilized for the encapsulation of different types of stem cells.
|
Stem Cell Type |
Biomaterial |
|
Mesenchymal stem cells |
Collagen-Fibrin [22], CH-GP-HEC [25], PHEMA-APMA-PAMAM [45], L-PDMA-PNIPAAm [46], PEG-PNIPAAm [26], Alginate [23], PEGDA [49], Fibrin [34], Gelatin-HA [27], PPS-b-PDMA-b-PNIPAAm [30], Alginate-Gelatin [16], PEG-4MAL-IFN-γ [53], HA-MAP [18][40] |
|
Bone marrow mesenchymal stem cells |
PNIPAAm-PHEMA-AOLA-PEGMA-PFO [33], CS-MA-PEGDA [24], Alginate-g-PNIPAAm [21] |
|
Wharton’s jelly mesenchymal stem cells |
Poloxamer 407-SAP [50] |
|
BMP-2 transduced bone marrow mesenchymal stem cells |
GelMA [20] |
|
Brain-derived neurotrophic factor-producing mesenchymal stem cells |
Alginate (ultrahigh viscous) [42] |
|
Adipose-derived stem cells |
Gelan gum-HA [15], Poloxamer 407 [51], Gelan gum [43], PNIPAAm-SWCNTs [39], Alginate [19], PEG [17] |
|
Telomerase-immortalized human adipose-derived stem cells |
Gelatin-Methacrylamide [29] |
|
Cardiosphere-derived cells |
|
|
Human cardiac stem cells |
PNIPAAm-PAA [37] |
|
Neural stem cells-dental pulp stem cells |
|
|
Induced pluripotent stem cell-derived neural progenitor cells |
CS-bFGF [32] |
|
Human embryonic stem cell derived-neural stem cells |
HA [44] |
|
Retinal stem cell-derived rods and neural stem and progenitor cells |
HA and methylecelluse [41] |
|
Dental pulp stem cells |
Poloxamer 407 [28] |
|
Human placenta-derived mesenchymal stem cells |
Alginate [31] |
|
Multipotent adult progenitor cells |
Alginate [52] |
|
Mouse embryonic stem cells |
PEG-fibrinogen [38] |
AOLA: acrylate-oligolactide; APMA: N-(3-Aminopropyl)methacrylamide; b: block; bFGF: basic fibroblast growth factor; BMP-2: Bone morphogenetic protein 2; CH: chitosan; CS: chondroitin sulfate; g: graft; GelMA: gelatin methacrylate; GP: glycerophosphate; HA: hyaluronic acid; HEC: hydroxyethyl cellulose; IFN-γ: interferon gamma; L: laponite; MA: methacrylate; MAP: mussel adhesive protein; OTMC: oligo(trimethylene carbonate); PAMAM: polyamidoamine; PDMA: poly(dimethylacrylamide); PEG: poly(ethylene glycol); PEGDA: poly(ethylene glycol) diacrylate; PEGMA: methacrylate-poly(ethylene glycol); PFO: perfluorooctane; PHEMA: poly(2-hydroxyethyl methacrylate); PNIPAAm: poly(N-isopropylacrylamide); PPAA: polypropylacrylic acid; PPS: poly[(propylene sulfide; SAP: sodium ascorbyl phosphate; SWCNTs: single-wall carbon nanotubes; 4MAL: four-arm maleimide.
The methodology, and particularly the physicochemical properties of the biomaterial that creates the cell envelope, are important for providing the appropriate niche, thus maintaining cell protection without losing the functional properties and benefits for cell therapy. There is a great variety of encapsulation techniques and gelation processes (Table 3) that have been thoroughly discussed in previous articles [54][55][56][57].
Table 3. Key encapsulation and gelation methodologies.
|
Encapsulation Methods |
Cross-Linking (Gelation) Methods |
|
|
Lithography · Photolithography · Soft Lithography |
Chemical cross-linking · Photopolymerization · Enzymatic reaction · Click reaction · Chemical copolymerization |
Physical cross-linking · Thermal · Electrostatic interaction · Phase inversion · Self-assembling peptides |
|
Extrusion · Electrospraying · Electrospinning |
||
|
Microfluidics · Droplet · Microfiber |
||
|
Bioprinting · Inkjet · Extrusion · Laser-assisted · Stereolithography |
||
|
Emulsification |
||
|
Cryogelation |
||
Encapsulation technology has greatly advanced due to the parallel advancement of material and microfluidics science, giving rise to microencapsulation that can allow even single-cell encapsulation [58], thus practically giving the opportunity to “dress up” individually the stem cells depending on the application.
2. Hydrogels
Hydrogels are materials that swell in water while retaining a substantial fraction of water within their structures [59]. They were first reported in 1960, by Wichterle and Lim, in their study of poly(2-hydroxyethyl methacrylate) (PHEMA) gel, which is used even today in soft contact lenses [60][61]. Since then, hydrogels have been widely studied and used in a great variety of biomedical applications [62], due to their close resemblance to natural living tissue. High water content, convenient porosity and soft structure are properties of hydrogels that enable them to simulate the physical characteristics of the extracellular matrix (ECM) more closely than any other class of synthetic biomaterials [63].
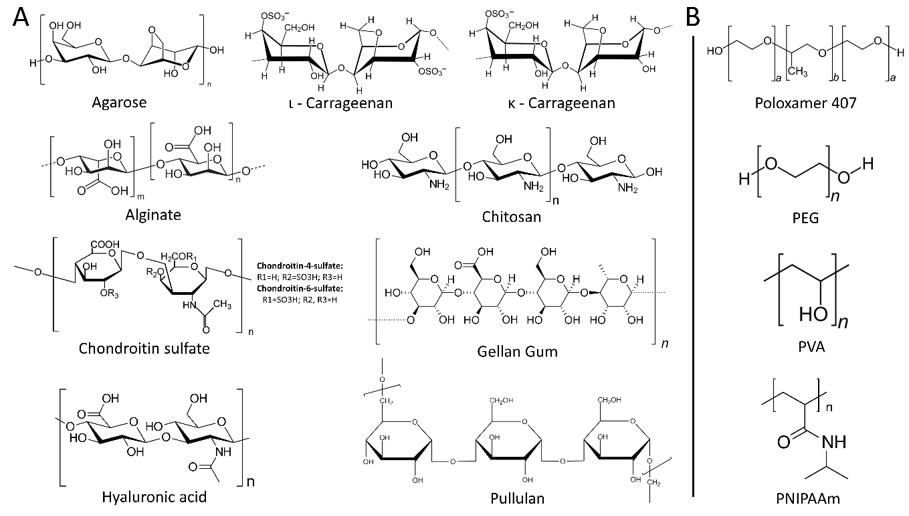
Hydrogels are formed by the cross-linking of polymer chains through either physical or chemical means and can be made of naturally occurring polymers, such as collagen or chitosan, or synthetic polymers like polyethylene glycol (PEG) (Figure 2) [64].
Figure 2. Chemical structures of natural (A) and synthetic (B) hydrogels.
The low interfacial tension between the hydrogels and the environmental fluids results in a low protein adsorption rate and cell adhesion, making them highly biocompatible. Moreover, the plasticity of hydrogels reduces the mechanical irritation on the surrounding area and provides a high degree of permeability to nutrients and metabolites [65]. There are many different ways that hydrogels may be classified, based on the polymer source, the polymeric composition, the type of cross-linking, their configuration or physical appearance [66]. In this enrty, hydrogels are categorized based on their origin.
2.1. Natural Hydrogels
2.1.1. Polysaccharides
Agarose
Agarose is a linear polymer consisting of repeating units of agarobiose, a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose, and it is extracted from the cell wall of a group of red algae (Rhodophyceae) [67].
In 2004, agarose was first used in stem cell encapsulation and proved to be a promising biomaterial, as stem cells could remain alive throughout the process and could differentiate into hematopoietic progenitors [68]. Since then, agarose has been used in numerous stem cell encapsulation studies, either alone or in combination with extracellular matrix proteins, such as collagen and fibrin. Batorsky et al. encapsulated human mesenchymal stem cells (hMSCs) in collagen–agarose and reported that cell viability remained high and their differentiation capacity was regulated by the collagen concentration; the higher its concentration, the greater the differentiation capacity towards the osteogenic lineage [69]. On the other hand, Sakai et al. used agarose alone as an encapsulation stem cell material and showed once again that agarose could permit stem cell proliferation and differentiation [70]. Finally, in a recent study, Benavente-Babace et al. studied the egress of therapeutically relevant cells, such as hMSCs, using a microfluidic device to produce monodisperse agarose microcapsules and concluded that agarose can be an excellent candidate for the encapsulation and delivery of mesenchymal stem cells (MSCs) in cell-based strategies of tissue regeneration [71].
Alginate
Alginate is an unbranched heteropolysaccharide of 1–4 glycosidically linked β-D-Mannuronic (M) and α-L-Guluronic (G) acids in varying composition and sequences, distributed widely in the cell walls of brown algae. In aqueous solutions and in the presence of divalent cations, such as Ca2+, they form gels through ionic cross-linking.
When it comes to stem cell encapsulation using alginate as a starting biomaterial, it is preferable not to use it alone but in combination with other polymers. Alginate alone forms dense hydrogels, it has low biodegradability and high stiffness and it blocks cellular growth due to the lack of cell adhesion molecules [72]. Rezaei et al. compared alginate–gelatin and alginate-alone hydrogels and found that the MSC growth rate was significantly greater in the mixed hydrogels [73]. For the same reason, Steiner et al. also used alginate–gelatin hydrogels for the encapsulation of MSCs and showed that not only the cell viability was unaltered but also that these scaffolds could promote angiogenesis in an in vivo model [16]. Nonetheless, alginate alone seems to have some positive effects, depending on the application. For instance, Al-Jaibaji et al. showed that the encapsulation of multipotent adult progenitor cells in alginate hydrogels could induce a wound-healing reaction in corneal stromal cells, through the secretion of soluble factors by the progenitor cells [52].
Carrageenan
Carrageenan is a sulfated polysaccharide that is extracted from red marine algae and it is comprised of either repeating disaccharide units of 4-linked β-D-galactopyranose (G-unit) and α-D-galactopyranose or 4-linked 3,6-anhydrogalactose, with a variable portion of sulfalite groups. According to the position and number of sulfate groups, carrageenans are divided into three families: κ, ι, and λ, corresponding to one, two, and three sulfate groups per disaccharide. In aqueous solutions and in the presence of cations, both κ- and ι-carrageenans easily form, on cooling, thermo-reversible gels [74][75].
Regarding carrageenan, there is only one reference in the literature in which it is used for mesenchymal stem cell encapsulation. In this study, Thakur et al. reported that upon encapsulation, stem cell viability remained high, no alterations in cell morphology, spreading and metabolic activities were observed and this biomaterial could be used for cartilage regeneration [76].
Chitosan
Chitosan is a natural, non-toxic, semi-crystalline, cationic polysaccharide with biocompatibility and structure similar to that of glycosaminoglycans. It is obtained industrially by hydrolyzing the amino-acetyl groups of chitin (primary component of cell walls in fungi, the exoskeletons of arthropods, such as crustaceans and insects, the radulae of mollusks, cephalopod beaks and the scales of fish and lissamphibians) by an alkaline treatment [77].
Due to its similarity to glycosaminoglycans, chitosan-based hydrogels are widely used in cartilage tissue engineering [78][79][80]. Naderi-Meshkin et al. showed that the encapsulation of MSCs in chitosan-based hydrogel could sustain cell viability and could promote differentiation towards the chondrogenic pathway [25]. Chitosan is also used in combination with other biomaterials, such as hyaluronan, and it has been shown that this combination can support cell viability, proliferation of adipose-derived stem cells and it can be a good candidate for tissue engineering [81].
Chondroitin Sulfate (CS)
CS is a sulfated glycosaminoglycan composed of chains of unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid and N-acetyl-D-galactosamine. It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities; chondroitin 4-sulfate (C4S or CSA) and chondroitin 6-sulfate (C6S or CSC) are two of the most common forms [82]. CS is an important structural component of cartilage and provides much of its resistance to compression. There are a number of different sources of CS; in the nutraceutical market, the main sources are bovine, shark and porcine cartilage [83].
CS has been proven to be a good candidate for applications that include neural stem cells. Karumbaiah L et al. used hydrogels containing CS for the encapsulation of neural stem cells and found that this biomaterial could regulate stem cell self-renewal and could highly absorb significant factors for the survival of neural stem cells [84]. Similarly, Liu C et al. observed that CS is a promising biomaterial for the treatment of spinal cord injuries, as it favors neural stem cells to differentiate into neurons, while at the same time, it inhibits astrocyte development, an unwanted side effect during the process of neural stem cell differentiation [48].
Gellan Gum
Gellan gum is an anionic heteropolysaccharide secreted by the bacteria Sphingomonas elodea (formerly Pseudomonas elodea) [85]. It is composed by tetrasaccharide repeating units consisting of 1,4-α-L-rahmnose, 1,3-β-D-glucose, 1,4-β-D-glucuronic acid and 1,4-β-D-glucose [86].
Although not widely used, there are a few references to its application in stem cell encapsulation. Cerqueira et al. used gellan gum-based hydrogel to encapsulate adipose-derived stem cells (ADSCs) and microvascular endothelial cells and they showed that this structure could promote wound healing and angiogenesis in an in vivo model [15]. In the same manner, Gomes et al. encapsulated in gellan gum-based hydrogel two different types of cells, ADSCs and olfactory ensheathing cells, and showed an increased in vitro growth rate and great motor improvements in an in vivo model of spinal cord injury [43]. Additionally, modified gellan gum-based hydrogels have shown promising results, again, in the field of neural tissue engineering [87].
Hyaluronic Acid
Hyaluronic acid (HA) is a relatively simple glycosaminoglycan present in mammals, characterized by repeating disaccharide units made up of 1,4-β-D-glucuronic acid and 1,3-β-N-acetyl-D-glucosamine [88].
HA is naturally found in soft tissues, such as the cartilage [89], and thus, previous studies have shown that it can successfully support chondrogenesis using MSCs [27][90][91]. Apart from its use in soft tissue engineering, HA has been also used in other systems. Ballios et al. reported that hyaluronan-based hydrogel could promote retinal stem cell survival in the retina, through interactions between the hydrogel and the stem cells, significantly improve visual function and the neural stem and progenitor cell state [41]. In addition, HA has been also used in combination with growth factors that promote stem cell differentiation, offering a useful tool for the in situ differentiation of pluripotent stem cells [41][92].
Pullulan
Pullulan is an extracellular and unbranched water-soluble homopolysaccharide, consisting of a-(1–6) linkages of a-(1–4)-linked maltotriose units, and is secreted primarily by strains of the fungus Aureobasidium pullulans. Due to its unique linkage pattern, pullulan has distinctive physical traits, such as structural flexibility and solubility; unlike other polysaccharides, it has great film and fiber forming capabilities that resemble those of synthetic polymers. It is biodegradable and highly water-soluble in its underivatized form [83][93][94].
Although pullulan is widely used in other applications, but not so far for stem cell encapsulation, a recent study investigated its properties and showed that it could be a good candidate for stem cell encapsulation [95].
2.1.2. Proteins
Collagen
Collagen is the major component of the extracellular matrix of connective tissues and it is responsible for their structure and functions. The collagen family consists of 28 distinct types that are distinguished by Roman numbers (I-XXVIII), following the chronological order of their discovery. All collagens are trimers, consisting of at least one stiff, rod-like domain of varying length, called collagenous domains and non-collagenous domains. The number and structure of these domains depend on the specific type of collagen [96].
Collagen is composed of a triple helix, having a divergent protein composition, mainly due to its high hydroxyproline content. It can be extracted from a variety of sources; it offers low immunogenicity, good permeability, biocompatibility and biodegradability, but relatively modest mechanical properties. To overcome this problem, various practices are employed, such as cross-linking by chemical or physical means and modifications with other polymers or inorganic materials [97].
Injectable collagen–phenolic hydroxyl hydrogels, capable of controlling a wide range of physicochemical properties, such as stiffness and degradability, were tested on the formation of vascularized engineered tissue graft by bone marrow-derived mesenchymal stem cells (BMSCs) in vivo. The results were promising as the hydrogel not only improved the long-term differentiation of the transplanted BMSCs into osteoblasts but also increased the number of adipocytes inside the vascularized engineered tissue after one month of implantation in a mouse [98].
Using a novel microfluidic device to encapsulate neural stem cells and dental pulp stem cells within an alginate–collagen hydrogel resulted in the survival of both cell types for up to three weeks in culture. Moreover, it was observed that the stem cells preserved their multipotency upon selective release from the microcapsules [47].
Elastin
Elastin is a highly elastic mammalian protein found in connective tissue that possesses the ability to resume the initial shape after the use of stretching or contracting mechanical force. It is rich in hydrophobic amino acids such as glycine and proline, which form mobile hydrophobic regions bounded by cross-links between lysine residues [99]. Elastin-based biomaterials are suitable for repairing elastic tissues, as they can improve local elasticity and support cellular interaction and signaling. Several studies that combine elastin hydrogels with MSCs have demonstrated their ability to also regenerate non-elastic tissue [100].
MSCs differentiation can be controlled by their immediate environment as it has been proven that tissue elasticity can influence their inclination towards specific lineages and phenotypes, thus making elastin hydrogels an ideal stem cell encapsulation candidate for specific applications [101]. Due to the growing accessibility of the elastin precursor, tropoelastin, there is increased research interest in stem cell encapsulation using elastin-based hydrogels [102].
Elastin-Like Protein and Hyaluronic Acid
The major limitation of HA hydrogels is the increased mechanical stiffness which is observed when there is also an increase in the concentration of HA [91][103][104]. This obstacle has been overcome by combining HA with elastin-like protein (ELP). Zhu et al. combined ELP with HA and created a new hydrogel for the encapsulation of bovine chondrocytes. They found that the mechanical stiffness of this biomaterial remained unchanged, even if the concentration of HA was altered. In addition, they observed that HA in varying concentrations could affect the proliferation rate of chondrocytes, their expression signatures and, ultimately, their differentiation fate. Zhu et al. concluded that the ELP–HA combination could enable the creation of soft hydrogels, ideal for chondrocyte proliferation and cartilage differentiation [105]. In another study by the same research team, it was shown that the ELP–HA combination could protect encapsulated MSCs from shredding when passed through a needle. It could also be rapidly reformed into its original shape, enabling MSCs to be homogenously dispersed into a 3D environment. In addition, they observed that this new biomaterial could enhance the survival of MSCs and prolong their culturing even up to three weeks after the injection. Finally, they reported that the differentiational capacity of encapsulated MSCs remained unaltered, suggesting that ELP–HA hydrogels might be excellent candidates for stem cell transplantations and tissue regeneration [106].
Fibrin
Fibrin monomers are naturally produced during the degradation of fibrinogen by the thrombin protease; they then polymerize and ultimately form fibrin networks with the help of other coagulation factors to prevent blood loss [102]. Fibrin, apart from its essential role in blood clot formation, is also used in the field of stem cell bioengineering. Lalegül-Ülker Ö et al. found that the encapsulation of MSCs in fibrin microbeads and their administration in rats could greatly regenerate muscle tissue injuries [34]. Fibrin-based hydrogels have been also found to be good candidates for the development of bone formation from MSCs. Heo DN et al. found that the encapsulation of MSCs in fibrin-based hydrogels and their supplementation with endothelial cells could further promote bone tissue formation [22]. These studies suggest that fibrin is a promising biomaterial in the field of muscle and bone tissue regeneration using MSCs.
Gelatin
Gelatin is a protein that is obtained when collagen is disintegrated into smaller parts through hydrolytic degradation. At temperatures exceeding 40 °C, gelatins are soluble in water, but upon cooling, they form transparent gels. Since gelatin derives from collagen, a natural source found in many organisms, it possesses several advantages; it does not cause antigenicity, it can be totally degradable in vivo, and its physicochemical properties can be regulated. Another crucial benefit of gelatin is the fact that it is presented with many functional side groups that enable it to be chemically cross-linked with other compounds, such as drugs, thus making it an excellent candidate for a drug delivery vehicle. Nonetheless, gelatins are not stable in the human body due to their low melting point and, therefore, they need to be stabilized by chemical cross-linking prior to their use [107].
In a previous study, Tzouanas SN et al. found that the gelatin loading and size of the microparticles could regulate MSC viability; the smaller the loading and size, the greater cell viability. In addition, they found that the interaction between the MSCs and the gelatin microparticles could initiate the differentiation process towards the osteogenic lineage, making these microparticles excellent candidates for tissue engineering [108]. In the same manner, Aparnathi MK and Patel JS showed that biodegradable, methacrylated gelatin gel could serve as a great scaffold for ADSC encapsulation, as it would not affect the osteogenic capacity of the stem cells [109]. The potential of gelatin-based microparticles to be used in stem cell encapsulation has also been shown in a recent study, in which MSCs could retain their viability and differentiation capacity inside colloidal gelatin microgels [110]. Taken together, these findings show that gelatin is an excellent biomaterial for stem cell encapsulation, as it is nontoxic for stem cells and it may promote their differentiation.
Keratin
Keratin is not a single substance but rather a complex formation that includes several types of keratin, keratin filament-associated proteins and enzymes, and it is produced by epithelial cells. It is highly resistant to degradation by enzymes, such as pepsin and trypsin, insoluble in aqueous solutions and can be found in tissues such as skin, hair and nails [111]. Due to its resistance in degradation, keratin-based scaffolds seem promising in tissue engineering, as they are slowly degraded, while collagen-based hydrogels are easily degraded. Barati D et al. showed that the use of keratin hydrogels in encapsulating MSCs could promote their differentiation into osteogenic and chondrogenic lineages, with a similar efficiency as in gelatin-based hydrogel, while having the advantage of not being easily degraded, thus making them an excellent candidate for a delivery system [112].
Silk Fibroin
Fibroin is an insoluble protein present in silk, mainly produced by the larvae of Bombyx mori, with an amino acid composition primarily of glycine, alanine and serine. Silk is composed of β-sheet structures, permitting tight packing of stacked sheets, granting the strength and resilience of silk fibers. Silk fibroin fibers consist of two proteins (a light and heavy chain) linked by a single disulfide bond in a 1:1 ratio. These proteins are coated with a family of hydrophilic proteins called sericins that are removed during the silk fibroin isolation process. The unique features of silk (structure, biocompatibility, versatile morphologies, genetic modification, thermal stability and controllable degradation) classify it as a favorable biomaterial for various clinical applications [113].
In a recent study, Patil and Singh were the first to demonstrate that silk fibroin–alginate beads can be used to encapsulate hMSCs at various cell densities, using an innovative, cell-compatible cross-linking method that allows for simultaneous encapsulation. In addition, by including carboxyl and phosphate groups into the beads, they showed that that hMSCs were able to grow, proliferate and differentiate into osteogenic and chondrogenic lineages without the need for differentiation media [114]. In another recent study, Hasturk et al. fabricated silk fibroin-based hydrogels which were cross-linked with either tyramine-substituted silk fibroin or gelatin; they then encapsulated hMSCs and found that these biomaterials could significantly improve the cell morphology and metabolic activity [115].
This entry is adapted from the peer-reviewed paper 10.3390/pr9010011
References
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Mol. Life Sci. 2020, doi:10.1007/s00018-020-03454-6.
- Paspaliaris, V.; Kolios, G. Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019, 2019, 1730978, doi:10.1155/2019/1730978.
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10, doi:10.1159/000345615.
- Sumer, H.; Liu, J.; Roh, S. Mesenchymal Stem Cells and Regenerative Medicine. Stem Cells Int. 2018, 2018, 9810972, doi:10.1155/2018/9810972.
- Tao, Y.C.; Wang, M.L.; Chen, E.Q.; Tang, H. Stem Cells Transplantation in the Treatment of Patients with Liver Failure. Stem Cell Res. Ther. 2018, 13, 193–201, doi:10.2174/1574888x13666180105123915.
- Lee, J.H.; Park, H.J.; Kim, Y.A.; Lee, D.H.; Noh, J.K.; Kwon, C.H.; Jung, S.M.; Lee, S.K. Differentiation and major histocompatibility complex antigen expression in human liver-derived stem cells. Proc. 2012, 44, 1113–1115, doi:10.1016/j.transproceed.2012.03.008.
- Wang, Y.; Tian, M.; Wang, F.; Heng, B.C.; Zhou, J.; Cai, Z.; Liu, H. Understanding the Immunological Mechanisms of Mesenchymal Stem Cells in Allogeneic Transplantation: From the Aspect of Major Histocompatibility Complex Class I. Stem Cells Dev. 2019, 28, 1141–1150, doi:10.1089/scd.2018.0256.
- Galvez-Martin, P.; Martin, J.M.; Ruiz, A.M.; Clares, B. Encapsulation in Cell Therapy: Methodologies, Materials, and Clinical Applications. Pharm. Biotechnol. 2017, 18, 365–377, doi:10.2174/1389201018666170502113252.
- Kühtreiber, W.M.; Lanza, R.P.; Chick, W.L. Cell Encapsulation Technology and Therapeutics; Springer Science & Business Media: New York, NY, USA, 1999.
- Aeron, G.; Shiwangi, M. Immobilization and microencapsulation. Adv. Res. Biotechnol. 2017, 2, 1–4.
- Hashemi, M.; Kalalinia, F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015, 143, 139–146, doi:10.1016/j.lfs.2015.11.007.
- Chang, T.M. Semipermeable microcapsules. Science 1964, 146, 524–525, doi:10.1126/science.146.3643.524.
- Kanda, P.; Alarcon, E.I.; Yeuchyk, T.; Parent, S.; De Kemp, R.A.; Variola, F.; Courtman, D.; Stewart, D.J.; Davis, D.R. Deterministic Encapsulation of Human Cardiac Stem Cells in Variable Composition Nanoporous Gel Cocoons To Enhance Therapeutic Repair of Injured Myocardium. ACS Nano 2018, 12, 4338–4350, doi:10.1021/acsnano.7b08881.
- Santos-Vizcaino, E.; Orive, G.; Pedraz, J.L.; Hernandez, R.M. Clinical Applications of Cell Encapsulation Technology. Methods Mol. Biol. 2020, 2100, 473–491, doi:10.1007/978-1-0716-0215-7_32.
- Cerqueira, M.T.; Da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Gellan gum-hyaluronic acid spongy-like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl. Mater. Interfaces 2014, 6, 19668–19679, doi:10.1021/am504520j.
- Steiner, D.; Lingens, L.; Fischer, L.; Kohn, K.; Detsch, R.; Boccaccini, A.R.; Fey, T.; Greil, P.; Weis, C.; Beier, J.P.; et al. Encapsulation of Mesenchymal Stem Cells Improves Vascularization of Alginate-Based Scaffolds. Tissue Eng. Part A 2018, 24, 1320–1331, doi:10.1089/ten.TEA.2017.0496.
- Young, S.A.; Flynn, L.E.; Amsden, B.G. Adipose-Derived Stem Cells in a Resilient In Situ Forming Hydrogel Modulate Macrophage Phenotype. Tissue Eng. Part A 2018, 24, 1784–1797, doi:10.1089/ten.TEA.2018.0093.
- Park, T.Y.; Jeon, E.Y.; Kim, H.J.; Choi, B.H.; Cha, H.J. Prolonged cell persistence with enhanced multipotency and rapid angiogenesis of hypoxia pre-conditioned stem cells encapsulated in marine-inspired adhesive and immiscible liquid micro-droplets. Acta Biomater. 2019, 86, 257–268, doi:10.1016/j.actbio.2019.01.007.
- Leslie, S.K.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Production of osteogenic and angiogenic factors by microencapsulated adipose stem cells varies with culture conditions. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1857–1867, doi:10.1002/jbm.b.34527.
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell. Res. Ther. 2019, 10, 254, doi:10.1186/s13287-019-1350-6.
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O'Doherty, M.; Chalanqui, M.; Kelly, D.J.; Haut-Donahue, T.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Biosci. 2017, 17, doi:10.1002/mabi.201700118.
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356, doi:10.1016/j.actbio.2019.02.046.
- Mandal, S.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Warrier, S. Encapsulated human mesenchymal stem cells (eMSCs) as a novel anti-cancer agent targeting breast cancer stem cells: Development of 3D primed therapeutic MSCs. J. Biochem. Cell Biol. 2019, 110, 59–69, doi:10.1016/j.biocel.2019.02.001.
- Rogan, H.; Ilagan, F.; Yang, F. Comparing Single Cell Versus Pellet Encapsulation of Mesenchymal Stem Cells in Three-Dimensional Hydrogels for Cartilage Regeneration. Tissue Eng. Part A 2019, 25, 1404–1412, doi:10.1089/ten.TEA.2018.0289.
- Naderi-Meshkin, H.; Andreas, K.; Matin, M.M.; Sittinger, M.; Bidkhori, H.R.; Ahmadiankia, N.; Bahrami, A.R.; Ringe, J. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol. Int. 2014, 38, 72–84, doi:10.1002/cbin.10181.
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176, doi:10.1016/j.actbio.2017.11.020.
- Liu, Y.; Shu, X.Z.; Prestwich, G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006, 12, 3405–3416, doi:10.1089/ten.2006.12.3405.
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. Mater. Sci. Mater. Med. 2015, 26, 153, doi:10.1007/s10856-015-5493-4.
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380, doi:10.1089/ten.TEA.2018.0237.
- Dollinger, B.R.; Gupta, M.K.; Martin, J.R.; Duvall, C.L. Reactive Oxygen Species Shielding Hydrogel for the Delivery of Adherent and Nonadherent Therapeutic Cell Types. Tissue Eng. Part A 2017, 23, 1120–1131, doi:10.1089/ten.tea.2016.0495.
- Fath-Bayati, L.; Ai, J. Assessment of mesenchymal stem cell effect on foreign body response induced by intraperitoneally implanted alginate spheres. Biomed. Mater. Res. A 2020, 108, 94–102, doi:10.1002/jbm.a.36795.
- McCrary, M.R.; Jesson, K.; Wei, Z.Z.; Logun, M.; Lenear, C.; Tan, S.; Gu, X.; Jiang, M.Q.; Karumbaiah, L.; Yu, S.P.; et al. Cortical Transplantation of Brain-Mimetic Glycosaminoglycan Scaffolds and Neural Progenitor Cells Promotes Vascular Regeneration and Functional Recovery after Ischemic Stroke in Mice. Healthc. Mater. 2020, 9, e1900285, doi:10.1002/adhm.201900285.
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020, 105, 56–67, doi:10.1016/j.actbio.2020.01.017.
- Lalegul-Ulker, O.; Seker, S.; Elcin, A.E.; Elcin, Y.M. Encapsulation of bone marrow-MSCs in PRP-derived fibrin microbeads and preliminary evaluation in a volumetric muscle loss injury rat model: Modular muscle tissue engineering. Cells Nanomed. Biotechnol. 2019, 47, 10–21, doi:10.1080/21691401.2018.1540426.
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957, doi:10.1021/acsami.6b04932.
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X.; Niu, H.; Li, X.; Xie, X.; Khan, M.; Guan, J. pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760, doi:10.1021/acsami.6b01374.
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749, doi:10.1021/acsnano.7b01008.
- Chang, S.; Finklea, F.; Williams, B.; Hammons, H.; Hodge, A.; Scott, S.; Lipke, E. Emulsion-based encapsulation of pluripotent stem cells in hydrogel microspheres for cardiac differentiation. Prog. 2020, 10.1002/btpr.2986, e2986, doi:10.1002/btpr.2986.
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lu, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688, doi:10.1016/j.biomaterials.2014.03.067.
- Park, T.Y.; Oh, J.M.; Cho, J.S.; Sim, S.B.; Lee, J.; Cha, H.J. Stem cell-loaded adhesive immiscible liquid for regeneration of myocardial infarction. Control. Release 2020, 321, 602–615, doi:10.1016/j.jconrel.2020.02.047.
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Rep. 2015, 4, 1031–1045, doi:10.1016/j.stemcr.2015.04.008.
- Scheper, V.; Hoffmann, A.; Gepp, M.M.; Schulz, A.; Hamm, A.; Pannier, C.; Hubka, P.; Lenarz, T.; Schwieger, J. Stem Cell Based Drug Delivery for Protection of Auditory Neurons in a Guinea Pig Model of Cochlear Implantation. Cell. Neurosci. 2019, 13, 177, doi:10.3389/fncel.2019.00177.
- Gomes, E.D.; Mendes, S.S.; Leite-Almeida, H.; Gimble, J.M.; Tam, R.Y.; Shoichet, M.S.; Sousa, N.; Silva, N.A.; Salgado, A.J. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials 2016, 105, 38–51, doi:10.1016/j.biomaterials.2016.07.019.
- Zarei-Kheirabadi, M.; Sadrosadat, H.; Mohammadshirazi, A.; Jaberi, R.; Sorouri, F.; Khayyatan, F.; Kiani, S. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. J. Biol. Macromol. 2020, 148, 1118–1129, doi:10.1016/j.ijbiomac.2020.01.219.
- Kumar, D.; Gerges, I.; Tamplenizza, M.; Lenardi, C.; Forsyth, N.R.; Liu, Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: A potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater. 2014, 10, 3463–3474, doi:10.1016/j.actbio.2014.04.027.
- Vickers, L.; Thorpe, A.A.; Snuggs, J.; Sammon, C.; Le Maitre, C.L. Mesenchymal stem cell therapies for intervertebral disc degeneration: Consideration of the degenerate niche. JOR Spine 2019, 2, e1055, doi:10.1002/jsp2.1055.
- Hidalgo San Jose, L.; Stephens, P.; Song, B.; Barrow, D. Microfluidic Encapsulation Supports Stem Cell Viability, Proliferation, and Neuronal Differentiation. Tissue Eng. Part C Methods 2018, 24, 158–170, doi:10.1089/ten.TEC.2017.0368.
- Liu, C.; Fan, L.; Xing, J.; Wang, Q.; Lin, C.; Liu, C.; Deng, X.; Ning, C.; Zhou, L.; Rong, L.; et al. Inhibition of astrocytic differentiation of transplanted neural stem cells by chondroitin sulfate methacrylate hydrogels for the repair of injured spinal cord. Sci. 2019, 7, 1995–2008, doi:10.1039/c8bm01363b.
- Aijaz, A.; Teryek, M.; Goedken, M.; Polunas, M.; Olabisi, R.M. Coencapsulation of ISCs and MSCs Enhances Viability and Function of both Cell Types for Improved Wound Healing. Mol. Bioeng. 2019, 12, 481–493, doi:10.1007/s12195-019-00582-3.
- Deng, Q.; Huang, S.; Wen, J.; Jiao, Y.; Su, X.; Shi, G.; Huang, J. PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell-mediated skin wound healing in mice. Stem Cell Res. Ther. 2020, 11, 143, doi:10.1186/s13287-020-01638-2.
- Kaisang, L.; Siyu, W.; Lijun, F.; Daoyan, P.; Xian, C.J.; Jie, S. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. Surg. Res. 2017, 217, 63–74, doi:10.1016/j.jss.2017.04.032.
- Al-Jaibaji, O.; Swioklo, S.; Gijbels, K.; Vaes, B.; Figueiredo, F.C.; Connon, C.J. Alginate encapsulated multipotent adult progenitor cells promote corneal stromal cell activation via release of soluble factors. PLoS ONE 2018, 13, e0202118, doi:10.1371/journal.pone.0202118.
- Garcia, J.R.; Quiros, M.; Han, W.M.; O'Leary, M.N.; Cox, G.N.; Nusrat, A.; Garcia, A.J. IFN-gamma-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials 2019, 220, 119403, doi:10.1016/j.biomaterials.2019.119403.
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Rev. Biomed. Eng. 2014, 16, 247–276, doi:10.1146/annurev-bioeng-071813-105155.
- Hasturk, O.; Kaplan, D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2019, 95, 3–31, doi:10.1016/j.actbio.2018.11.040.
- Kim, H.; Bae, C.; Kook, Y.M.; Koh, W.G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 51, doi:10.1186/s13287-018-1130-8.
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. Chemical gelling of hydrogels-based biological macromolecules for tissue engineering: Photo- and enzymatic-crosslinking methods. J. Biol. Macromol. 2019, 139, 760–772, doi:10.1016/j.ijbiomac.2019.08.047.
- Kamperman, T.; Karperien, M.; Le Gac, S.; Leijten, J. Single-Cell Microgels: Technology, Challenges, and Applications. Trends Biotechnol. 2018, 36, 850–865, doi:10.1016/j.tibtech.2018.03.001.
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Drug Del. Rev. 1993, 11, 1–35, doi:10.1016/0169-409X(93)90025-Y.
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118, doi:10.1038/185117a0.
- Lee, D.; Cho, S.; Park, H.S.; Kwon, I. Ocular Drug Delivery through pHEMA-Hydrogel Contact Lenses Co-Loaded with Lipophilic Vitamins. Rep. 2016, 6, 34194, doi:10.1038/srep34194.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Polym. J. 2015, 65, 252–267, doi:10.1016/j.eurpolymj.2014.11.024.
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. J. Pharm. Biopharm. 2000, 50, 27–46, doi:10.1016/s0939-6411(00)00090-4.
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351, doi:10.1016/S0142-9612(03)00340-5.
- Uludag, H.; De Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Drug Deliv. Rev. 2000, 42, 29–64, doi:10.1016/s0169-409x(00)00053-3.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. Adv. Res. 2015, 6, 105–121, doi:10.1016/j.jare.2013.07.006.
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, doi:10.3390/molecules21111577.
- Dang, S.M.; Gerecht-Nir, S.; Chen, J.; Itskovitz-Eldor, J.; Zandstra, P.W. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells 2004, 22, 275–282, doi:10.1634/stemcells.22-3-275.
- Batorsky, A.; Liao, J.; Lund, A.W.; Plopper, G.E.; Stegemann, J.P. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Bioeng. 2005, 92, 492–500, doi:10.1002/bit.20614.
- Sakai, S.; Hashimoto, I.; Kawakami, K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Bioeng. 2008, 99, 235–243, doi:10.1002/bit.21624.
- Benavente-Babace, A.; Haase, K.; Stewart, D.J.; Godin, M. Strategies for controlling egress of therapeutic cells from hydrogel microcapsules. Tissue Eng. Regen. Med. 2019, 13, 612–624, doi:10.1002/term.2818.
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized Alginate-Gelatin Hydrogel: A Favorable Matrix for Growth and Osteogenic Differentiation of Adipose-Derived Stem Cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737, doi:10.1021/acsbiomaterials.7b00188.
- Rezaei, S.; Shakibaie, M.; Kabir-Salmani, M.; Soltani Moghaddam, M.; Rezvani, M.; Shahali, M.; Naseri, M. Improving the Growth Rate of Human Adipose-Derived Mesenchymal Stem Cells in Alginate/Gelatin Versus Alginate Hydrogels. J. Biotechnol. 2016, 14, 1–8, doi:10.15171/ijb.1185.
- Popa, E.; Reis, R.; Gomes, M. Chondrogenic phenotype of different cells encapsulated in kappa-carrageenan hydrogels for cartilage regeneration strategies. Appl. Biochem. 2012, 59, 132–141, doi:10.1002/bab.1007.
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. Bioact. Compat. Polym. 2011, 26, 493–507, doi:10.1177/0883911511420700.
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 2016, 8, 12362–12372, doi:10.1039/c6nr02299e.
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. Microencapsul. 1997, 14, 689–711, doi:10.3109/02652049709006820.
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng. 2007, 13, 1905–1925, doi:10.1089/ten.2006.0175.
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396, doi:10.1016/j.biomaterials.2005.06.037.
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299, doi:10.1089/ten.TEB.2011.0077.
- Fan, M.; Ma, Y.; Mao, J.; Zhang, Z.; Tan, H. Cytocompatible in situ forming chitosan/hyaluronan hydrogels via a metal-free click chemistry for soft tissue engineering. Acta Biomater. 2015, 20, 60–68, doi:10.1016/j.actbio.2015.03.033.
- Baeurle, S.A.; Kiselev, M.G.; Makarova, E.S.; Nogovitsin, E.A. Effect of the counterion behavior on the frictional-compressive properties of chondroitin sulfate solutions. Polymer 2009, 50, 1805–1813, doi:10.1016/j.polymer.2009.01.066.
- Foot, M.; Mulholland, M. Classification of chondroitin sulfate A, chondroitin sulfate C, glucosamine hydrochloride and glucosamine 6 sulfate using chemometric techniques. Pharm. Biomed. Anal. 2005, 38, 397–407, doi:10.1016/j.jpba.2005.01.026.
- Karumbaiah, L.; Enam, S.F.; Brown, A.C.; Saxena, T.; Betancur, M.I.; Barker, T.H.; Bellamkonda, R.V. Chondroitin Sulfate Glycosaminoglycan Hydrogels Create Endogenous Niches for Neural Stem Cells. Chem. 2015, 26, 2336–2349, doi:10.1021/acs.bioconjchem.5b00397.
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a pseudomonas species: Production and basic properties. Environ. Microbiol. 1982, 43, 1086–1091.
- Jansson, P.E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Res. 1983, 124, 135–139.
- Koivisto, J.T.; Joki, T.; Parraga, J.E.; Paakkonen, R.; Yla-Outinen, L.; Salonen, L.; Jonkkari, I.; Peltola, M.; Ihalainen, T.O.; Narkilahti, S.; et al. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Mater. 2017, 12, 025014, doi:10.1088/1748-605X/aa62b0.
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Mater. 2009, 21, 3307–3329, doi:10.1002/adma.200802106.
- Morgelin, M.; Heinegard, D.; Engel, J.; Paulsson, M. The cartilage proteoglycan aggregate: Assembly through combined protein-carbohydrate and protein-protein interactions. Chem. 1994, 50, 113–128, doi:10.1016/0301-4622(94)85024-0.
- Nettles, D.L.; Vail, T.P.; Morgan, M.T.; Grinstaff, M.W.; Setton, L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Biomed. Eng. 2004, 32, 391–397, doi:10.1023/b:abme.0000017552.65260.94.
- Chung, C.; Mesa, J.; Randolph, M.A.; Yaremchuk, M.; Burdick, J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. Biomed. Mater. Res. A 2006, 77, 518–525, doi:10.1002/jbm.a.30660.
- Lee, J.; Lee, S.H.; Kim, B.S.; Cho, Y.S.; Park, Y. Development and Evaluation of Hyaluronic Acid-Based Hybrid Bio-Ink for Tissue Regeneration. Tissue Eng. Regen. Med. 2018, 15, 761–769, doi:10.1007/s13770-018-0144-8.
- San Juan, A.; Hlawaty, H.; Chaubet, F.; Letourneur, D.; Feldman, L.J. Cationized pullulan 3D matrices as new materials for gene transfer. Biomed. Mater. Res. A 2007, 82, 354–362, doi:10.1002/jbm.a.31062.
- Leathers, T.D. Biotechnological production and applications of pullulan. Microbiol. Biotechnol. 2003, 62, 468–473, doi:10.1007/s00253-003-1386-4.
- Luca, A.; Maier, V.; Maier, S.S.; Butnaru, M.; Danu, M.; Ibanescu, C.; Pinteala, M.; Popa, M. Biomacromolecular-based ionic-covalent hydrogels for cell encapsulation: The atelocollagen—Oxidized polysaccharides couples. Polym. 2017, 169, 366–375, doi:10.1016/j.carbpol.2017.04.046.
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Exp. Med. Biol. 2014, 802, 5–29, doi:10.1007/978-94-007-7893-1_2.
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, doi:10.3390/polym8020042.
- Kuo, K.C.; Lin, R.Z.; Tien, H.W.; Wu, P.Y.; Li, Y.C.; Melero-Martin, J.M.; Chen, Y.C. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015, 27, 151–166, doi:10.1016/j.actbio.2015.09.002.
- Rosenbloom, J. Elastin: Relation of protein and gene structure to disease. Investig. 1984, 51, 605–623.
- Ozsvar, J.; Mithieux, S.M.; Wang, R.; Weiss, A.S. Elastin-based biomaterials and mesenchymal stem cells. Sci. 2015, 3, 800–809, doi:10.1039/c5bm00038f.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689, doi:10.1016/j.cell.2006.06.044.
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. R. Soc. Interface 2014, 11, 20140817, doi:10.1098/rsif.2014.0817.
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421, doi:10.1016/j.biomaterials.2012.09.052.
- Martens, P.J.; Bryant, S.J.; Anseth, K.S. Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules 2003, 4, 283–292, doi:10.1021/bm025666v.
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140, doi:10.1016/j.biomaterials.2017.02.010.
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently Adaptable Elastin-Like Protein–Hyaluronic Acid (ELP–HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery. Funct. Mater. 2017, 27, doi:10.1002/adfm.201605609.
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38, doi:10.1021/bm990017d.
- Tzouanas, S.N.; Ekenseair, A.K.; Kasper, F.K.; Mikos, A.G. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. Biomed. Mater. Res. A 2014, 102, 1222–1230, doi:10.1002/jbm.a.35093.
- Aparnathi, M.K.; Patel, J.S. Biodegradable Gelatin Methacrylate Gel as a Potential Scaffold for Bone Tissue Engineering of Canine Adipose-Derived Stem Cells. Stem Cells 2016, 11, 111–119.
- Sung, B.; Krieger, J.; Yu, B.; Kim, M.H. Colloidal gelatin microgels with tunable elasticity support the viability and differentiation of mesenchymal stem cells under pro-inflammatory conditions. Biomed. Mater. Res. A 2018, 106, 2753–2761, doi:10.1002/jbm.a.36505.
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. Anat. 2009, 214, 516–559, doi:10.1111/j.1469-7580.2009.01066.x.
- Barati, D.; Kader, S.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Sawyer, R.H.; Jabbari, E. Synthesis and Characterization of Photo-Cross-Linkable Keratin Hydrogels for Stem Cell Encapsulation. Biomacromolecules 2017, 18, 398–412, doi:10.1021/acs.biomac.6b01493.
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Polym. Sci. 2007, 32, 991–1007, doi:10.1016/j.progpolymsci.2007.05.013.
- Patil, S.; Singh, N. Silk fibroin-alginate based beads for human mesenchymal stem cell differentiation in 3D. Sci. 2019, 7, 4687–4697, doi:10.1039/c9bm01000a.
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720, doi:10.1016/j.biomaterials.2019.119720.