The current knowledge and advancements on various nanoplatforms (NF) and the use of nanoparticles for successful cross of BBB to treat the brain-related disorders such as brain tumors, Alzheimer’s disease, Parkinson’s disease, and stroke.
1. Introduction
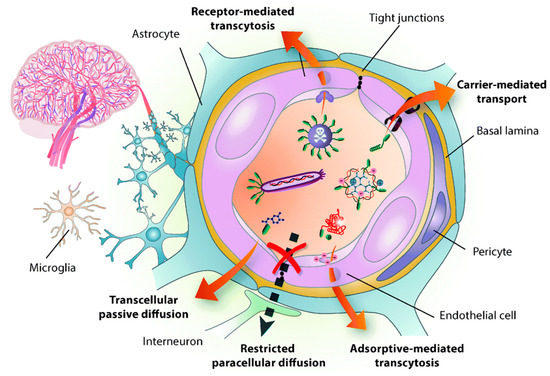
Disorders in the central nervous system (CNS) creates a potential impact on public health and have remained the leading cause of death, mainly in Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, and brain tumors [1,2,3,4]. However, the current strategies are very far from impressive to treat the CNS, owing to the restriction of BBB for transporting drugs to the brain [5]. As a result, almost 98% of the small-molecule drug and 100% of the macromolecular drugs are unable to enter the brain [6]. The BBB is a physiological structure of the blood vessels in the brain. It not only precisely regulates the entrance and discharge of ions, cells, and molecules between the blood and brain tissue, but it also has an important function in maintaining a microenvironment for reliable neuronal signaling [7]. The BBB is responsible for brain homeostasis and protection and is composed of pericytes (PCs), endothelial cells (ECs), a basement membrane, and astrocytes. ECs form the walls of the vessels through intermolecular tight junctions (TJs). The BBB can restrict the access of molecules into the brain and provides a natural shield against various toxins and infected cells from circulating blood, but it also limits the brain’s uptake of diagnostic and therapeutic agents, thus, reducing therapeutic efficiency [8,9]. An analysis of over 7000 drugs found that only 1% could penetrate the BBB and be active in the central nervous system (CNS) [7,10]. Therefore, the BBB is the main hindrance to noninvasive treatment of brain-related diseases (such as Parkinson’s disease, Alzheimer’s disease, schizophrenia, depression, and brain tumors) because the BBB restricts passage only to necessary substrates from the circulation to the brain tissue [11,12]. The detailed structure of BBB and transport mediated pathways was shown in . Other than nutrients, it was shown that small lipophilic molecules (most low-o500Da) are able to cross the BBB effectively and reach the brain [13]. Thereafter, many strategies have been developed to nonspecifically disrupt the BBB and, thus, allow the therapeutic agents to enter into the brain, but these strategies may also allow circulating toxins enter the brain from the blood. Therefore, numerous efforts have been attempted to develop novel strategies, which are able to deliver therapeutic drugs to CNS by crossing the BBB.
Figure 1. Structure of the blood brain barrier (BBB) and transport pathways across the BBB. Reproduced with permission from Reference [14].
2. BBB Penetrating Nanoplatforms (NFs) in Biomedical Applications
By understanding the structure of BBB and utilizing the beneficial advantages of surface modified-NPs, several NFs were successfully applied in various biomedical applications with significant outcomes. Thus, in here, we are more focused on the BBB penetrating NFs, specifically for brain tumor therapy, Alzheimer’s disease, Parkinson’s disease, and stroke applications.
2.1. BBB-Penetrating NPs for Brain Tumor Therapy
Malignant gliomas are primary brain tumors derived of glial origin, and 70% of glioma patients survive less than 15 months past diagnosis, even with surgical excision and/or chemo radiation therapy [
60,
61]. Unfortunately, radio therapy causes serious side effects such as post-radiation leuko-encephalopathy, nerve damage, hair loss, vomiting, infertility, and skin rash. As well, chemotherapy is also limited due to the toxic effects of the healthy cells, chemo resistance, and poor selectivity of anti-cancer drugs. Above all, BBB is the major limit for the delivery of chemotherapeutic agents that results in lower tumor accumulation of drug, tumor heterogeneity affecting sensitivity, and drug resistance [
62]. Thus, novel strategies to further improve the brain tumor diagnostics and therapeutics is highly desired. Over the advantages of nanotechnology, several drug molecules were successfully encapsulated into the nanocarrier systems and deliver to brain or facilitate penetration through the BBB, thereby overcoming the previous drug delivery chemotherapeutic issues to unreachable tumors, such as glioblastoma multiforme (GBM) [
63]. Subsequently, several kinds of nano-formulations were developed to load and deliver the hydrophilic and hydrophobic factors to the tumor site by crossing the BBB. For instance, prolonged half-life of Temozolomide (TMZ) was achieved around 13.4 h when it was encapsulated in the Chitosan-based NPs whereas a free drug having only a 1.8-h half-life [
64]. Drug-loaded albumin NPs were recently found to target SPARC (secreted protein acidic and rich in cysteine) and gp60 (glycoprotein 60), which are overexpressed in glioma and tumor vessel endothelia [
65]. Therefore, such pathways have been explored for use in brain-targeting biomimetic delivery. The albumin NPs also displayed enhanced BBB penetration, intra tumoral infiltration, and cellular uptake [
66]. Overall, NPs exhibit great potential in preclinical studies. Besides the passive targeting strategy, active targeting might be employed to further promote the accumulation of therapeutic drugs at the brain tumor site. Another rat brain model examined the encapsulation of methotrexate-transferrin complexes and coating of polysorbate 80 on poly-lactic-co-glycolic acid NPs, finding better BBB-penetration, lower organ toxicity, and higher anti-tumor activity as compared with non-targeting NPs [
67]. There is an upgraded need to further improve the compound solubility, stability, and reduce systematic toxicities of NPs.
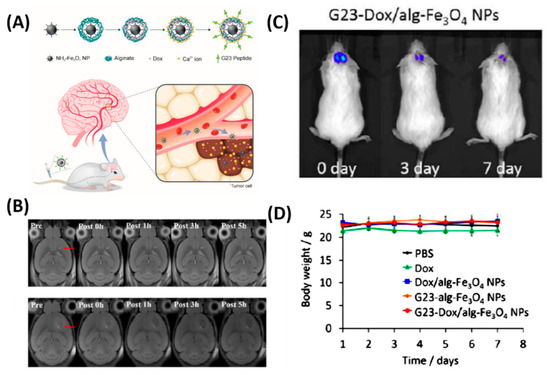
Besides the chemo delivery platform, diagnostic tools such as a high-resolution imaging system before surgery is highly important for GBM, which are characteristically invasive. For instance, gadolinium NPs used as a magnetic resonance (MR) contrast agent can penetrate the BBB and are taken up by the brain tumor parenchyma [
68]. To further achieve the therapeutic efficiency, diagnostic and chemotherapeutic platforms are attracted to monitor the tumor, especially for brain tumors. Thus, Cheng et al. synthesized a doxorubicin (DOX) nanocarrier composed of Fe
3O
4 NPs (particle size: 140 nm, zeta potential: −15 mV) and alginate, tagged with the BBB-permeating G23 peptides on the particle surface (G23-Dox/alg-Fe
3O
4 NPs) [
69]. Tumors (U87MG) significantly shrank (from ~50 mm
3 to a few mm
3) in mice treated with G23-Dox/alg-Fe
3O
4 NPs after being intravenously injected with NPs for five days, which was confirmed by contrast-enhanced T2-weighted MRI images (). In another study by Ni et al., it was demonstrated that the ANG/PEG-UCNPs nanoprobes targeted the glioblastoma efficiently via receptor mediated transcytosis [
22]. Moreover, these nanoprobes greatly offer a MR imaging and near-infrared to near-infrared (NIR-to-NIR) upconversion luminescence (UCL) fluorescence imaging to visualize the tumors, which exhibited excellent performance that the clinically used MRI contrasts.
Figure 3. In vivo anti-tumor activity of G23-Dox/alg-Fe
3O
4 NPs. (
A) Schematic representation of synthesis process and BBB penetrating Dox delivery. (
B) In vivo MRI contrast imaging abilities of alg-Fe
3O
4 NPs and G23-alg-Fe
3O
4 NPs. (
C) In vivo luminescence images show from U87MG-luc2 cells monitored using the IVIS imaging system after mice were intravenously injected with G23-Dox/alg-Fe
3O
4 NPs for seven days. (
D) Body weights of mice during the treatment. Reproduced with permission from Reference [
69].
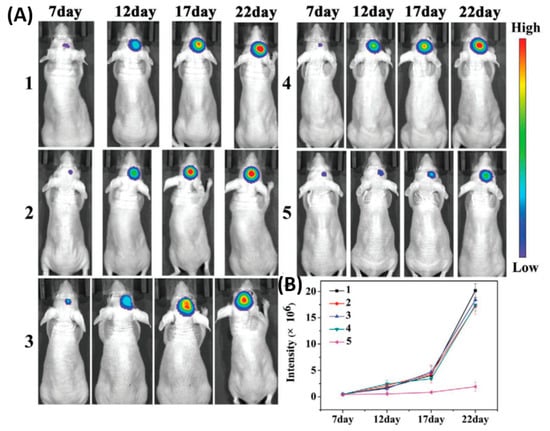
As shown in , the cell membrane camouflaged NPs exhibited a significantly higher accumulation at the tumor site by crossing the BBB than bare NPs, which was confirmed by stronger fluorescence signals that were observed in the brain of mice at 8 h. B16-PCL-ICG NPs could efficiently inhibit the glioma tumor growth under 808-nm laser irradiation mediated via PTT.
Figure 4. Cell membranes coated on ICG loaded nanoparticle (PCL-ICG) nanoparticles (NPs). (
A) Representative bioluminescence images of U87MG-Luc glioma-bearing mice in different groups: (1) phosphate buffered saline (PBS), (2) normal cell coated ICG loaded nanoparticle (COS7-PCL-ICG), (3) COS7-PCL-ICG + laser, (4) B16-PCL-ICG, and (5) B16-PCL-ICG + laser under 808-nm laser irradiation (1 W cm
−2, 5 min). CICG = 1 mg kg
−1. (
B) Quantitative fluorescence signal intensity in the brain. Reproduced with permission from Reference [
74].
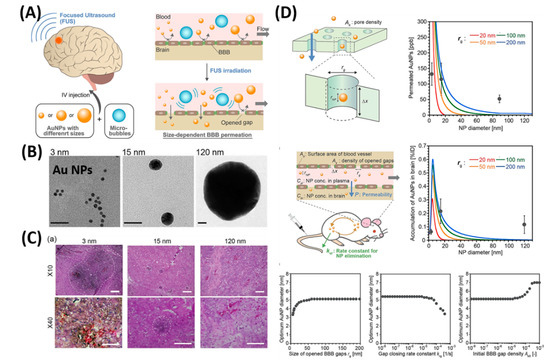
As shown in , Ohta et al. investigated the size dependent delivery of Au NPs (30 to 120 nm) into the brain by crossing the BBB assisted by FUS [
104]. In vivo experimental results reveal that smaller particles were not necessarily better for delivery systems, but the medium-sized Au NPs (15 nm) showed the highest delivery into the brain (2.2% ID via 0.7 MPa FUS) when compared to the smaller size (3 nm) and larger size of Au NPs (120 nm). The probable reason behind the size dependent permeability is mainly due to the competition between the permeation through BBB and excretion of particles from blood circulation. Experimental results exhibited that smaller NPs are preferable to deliver into the brain via BBB, but are quickly removed from the blood stream via kidneys. Besides, nose-to-brain delivery via intranasal administration of nano-formulations offers significant advantages such as easy penetration through the BBB and rapidly deliver the therapeutic drugs for the treatment of CNS disorders [
105,
106].
Figure 5. Focused ultrasound-induced blood–brain barrier opening strategy. (
A) Schematic representation of size-dependent nanoparticle (NP) delivery to the brain via a focused ultrasound (FUS). (
B) TEM images of 3, 15, 120-nm sized Au NPs. (
C) Distribution of Au NPs in mouse brains in vivo models. (
D) Kinetic modeling studies of FUS-assisted NPs delivery into the brain. Reproduced with permission from Reference [
104].
2.2. BBB-Penetrating NPs for Alzheimer’s Disease (AD)
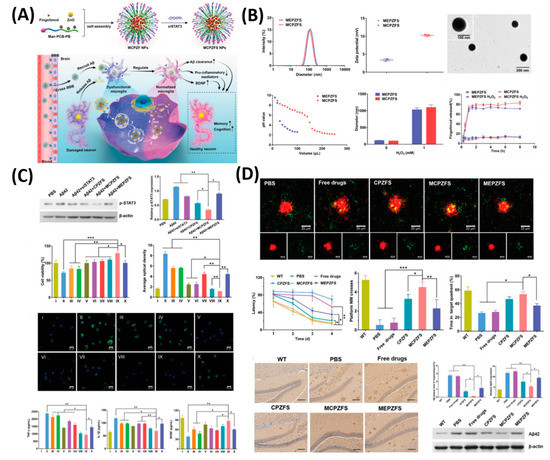
As shown in , as a proof-of-concept, 84 nm of MCPZF NPs was synthesized and it offers superior siRNA condensation, which was evidenced by gel-electrophoresis. Furthermore, modification of PCB could efficiently facilitate endosomal/lysosomal escape by protonation and perturbation. Thereafter, we studied the effect of present NPs on the inflammatory regulation of microglia by essaying the p-STAT3 protein levels and levels of pro-inflammatory cytokines. Results exhibited that the MCPZFS NPs could significantly inhibit the Aβ-induced cytotoxicity by increasing the production of Brain-derived neurotrophic factor (BDNF) and decreasing the levels of proinflammatory cytokines, which might be attributed due to the excellent properties of NPs by escaping the endosomal/lysosomal. Besides, intracellular distribution of Aβ and NPs in BV2 cells further proved the enhanced microglial phagocytosis on the present system. On the other hand, small interfering RNAs (siRNAs) show a promising platform to treat the AD by silencing BACE1. However, a lack of carrier systems to deliver the siRNA to the brain is limited. Thus, Zhou et al. very recently reported glycosylated “triple-interaction” stabilized polymeric siRNA nanomedicine (Gal-NP@siRNA) to target BACE1 in a transgenic AD mouse model [
118]. The results show the partial knockdown of BACE1 protein expression on the present NFs without noticeable side effects. These strategies indicated that Gal-NP@siRNA NFs has an excellent clinical translation potential for AD treatment owing to its stability, ease formulation, and successful BBB penetration.
Figure 6. Zwitterionic poly(carboxybetaine) (PCB)-based nanoparticle (MCPZFS NPs) for treating Alzheimer’s disease (AD). (
A) Schematic illustration of the MCPZFS NPs for AD. (
B) Characterization of the NPs. (
C) Effect of NPs on the inflammatory regulation of microglia and (
D) the effect of NPs on phagocytosis and degradation of Aβ by microglia. Data are presented as the mean ± SD. *
p < 0.05, **
p < 0.01, ***
p < 0.001. Reproduced with permission from Reference [
117].
2.3. BBB-Penetrating NPs for Parkinson’s Disease (PD)
PD is a progressive disease of the nervous system that affects a person’s movement, including writing and speaking. While the cause of the illness is still unknown, it is related to insufficient dopamine production by nerve cells in the brain. Currently, the most widely used strategy for PD treatment is dopamine replacement to improve motor function. To increase the dopamine concentration in the brain, direct dopamine infusion into the brain of PD animal models were reported but it has an unsuccessful end due to the fact that dopamine is not able to cross the BBB and, therefore, direct infusion is not possible, which results in behavioral abnormalities observed in animal models [
119,
120]. As a beneficial advantage of NPs, several nano carriers were developed to deliver the drugs to treat the PD efficiently. For instance, Huang et al. developed a neurotrophic factor gene (hGDNF, a plasmid for the human glial cell line)-loaded Polyamidoamine (PAMAM) and polyethyleneglycol (PEG) NPs modified by lactoferrin [
121]. Glial cell line-derived neurotrophic factor (GDNF) is the golden standard neurotrophic factor for PD therapy. However, it is unable to cross the BBB. Lactoferrin-conjugated PAMAM and PEG NPs could deliver GDNF across the BBB to exert a neuroprotective effect on dopaminergic neurons. Thereafter, to deliver the dopamine to the brain efficiently via BBB, Pahuja et al. developed dopamine-loaded PLGA NPs (DA-PLGA NPs) that crossed the BBB mainly in the substantia nigra and striatum (PD-altered regions) of 6-hydroxydopamine rats [
122]. In their study, DA-PLGA NPs prevent toxicity from bulk dopamine and provides a novel strategy to treat PD. Thereafter, other drugs like the ropinirole (RP) drug loaded into the PLGA NPs, were developed to demonstrate the drug delivery to the brain for treating PD with significant outcomes [
123].
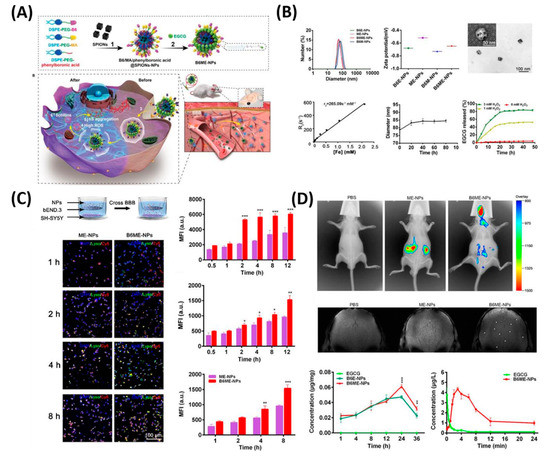
In another feature of PD pathogenesis is α-synclein (αS) aggregation. This αS Aggregation could be prevented by Epigallocatechin gallate (EGCG). However, it is very difficult to accumulate the EGCG in vivo models to the BBB. Therefore, Li et al. reported cell-addictive,” traceable, ROS-responsive NPs with dual targets for delivering an Epigallocatechin gallate (EGCG) in dopaminergic neurons for treating PD [
124]. As shown in , the amount of EGCG accumulation in PD lesions was significantly enhanced on the fabricated B6ME-NPs. Moreover, incorporated superparamagnetic iron oxide nanocubes (SPIONs) helps to trace the drug molecules via magnetic resonance imaging. Finally, released EGCG inhibits αS aggregation and reduces the toxicity of dopaminergic neurons.
Figure 7. Dual-target traceable nanodrug for Parkinson’s disease (PD) treatment. (
A) Schematic representation of synthesis of nanodrug and application for PD. (
B) Systematic characterization of dual-target traceable nanodrug (B6ME-NPs). (
C) Confocal microscopy (CSLM) and flow cytometry uptake studies to confirm the successful blood brain barrier (BBB) crossing. (
D) Fluorescence and magnetic resonance (MR) images of the mice model after 24 h of i.v. injection of the nanodrug. Data are presented as the mean ± SD. *
p < 0.05, **
p < 0.01, ***
p < 0.001. Reproduced with permission from Reference [
124].
2.4. BBB-Penetrating NPs for Stroke
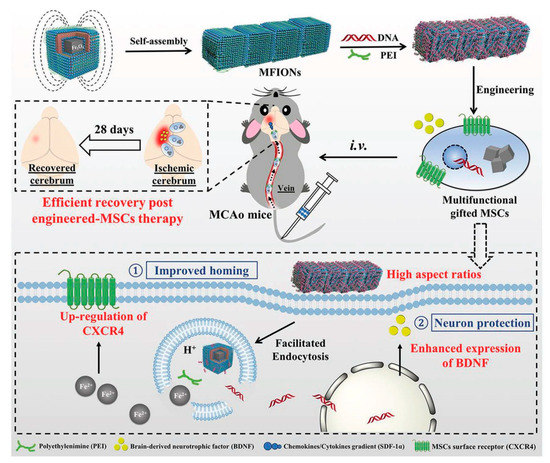
As shown in , the present platform offers a favorable cellular uptake and high stem cell gene delivery. Moreover, ferrous ions released from MFIONs can efficiently excite the upregulations of CXCRC4. Finally, high r2 relaxivity of MFIONS allow sensitive and non-invasive monitoring of MRI. However, most of the NP strategies suffer from shorter vascular circulation time, aggregation, and other undesirable catalytic reaction at active sites, which makes them limited for further clinical development. Recently, He et al. developed a bioactive zeolitic imidazolate framework-8–capped ceria nanoparticles (CeO
2@ZIF-8 NPs) for improving the therapeutic efficiency of ischemic stroke [
152]. The present nanoplatform offers improved BBB permeation, prolonged blood circulation times, and more accumulation in the brain makes them potential agents to inhibit the lipid peroxidation in the brain tissues and reduces the oxidative damage and apoptosis of neurons in the brain tissue. It also suppresses the inflammation and immune response-induced injuries by suppressing the activation of astrocytes and secretion of proinflammatory cytokines, thus, achieving satisfactory prevention and treatment in neuroprotective therapy. As known, the adhesion of neutrophils to endothelial cells triggers the initiation of inflammation in ischemia/reperfusion (I/R). Based on this concept, Dong et al., developed neutrophil membrane-coated NFs loaded with a Resollvin D2 (RvD2) drug to prevent neuroinflammation. This platform offers an enhancing resolution of inflammation during ischemic stroke therapy [
133].
Figure 8. Schematic representation of magnetosome-like ferrimagnetic iron oxide nanochains (MFION)-based fabrication of Mesenchymal stem cells (MSCs) for the recovery of post-ischemic stroke. Reproduced with permission from Reference [
151].
This entry is adapted from the peer-reviewed paper 10.3390/polym12123055