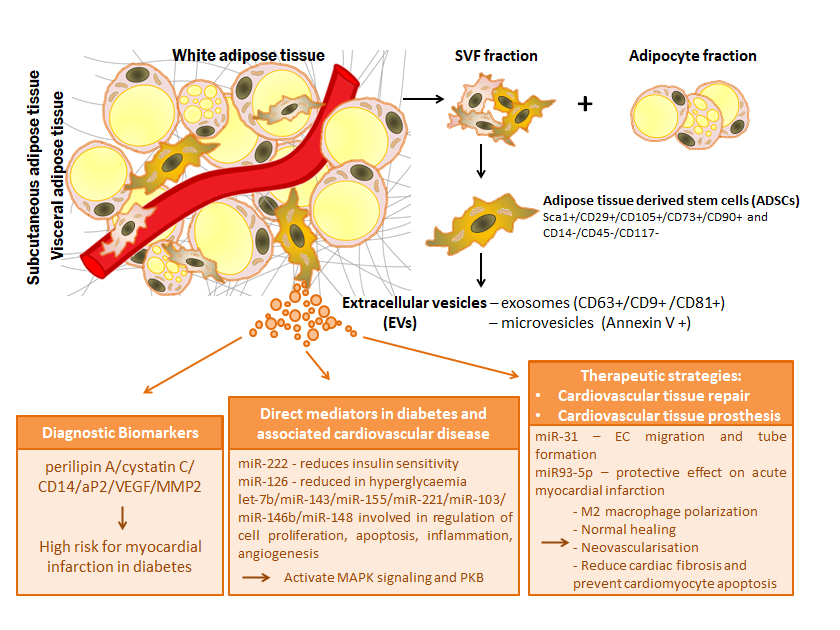
Adipose tissue-derived stem cells (ADSCs) are pluripotent mesenchymal stem cells found in relatively high percentages in the adipose tissue and able to self-renew and differentiate into many different types of cells. Extracellular vesicles (EVs), small membrane vesicular structures released during cell activation, senescence, or apoptosis, act as mediators for long distance communication between cells, transferring their specific bioactive molecules into host target cells. Metabolic syndrome and type 2 diabetes mellitus (T2DM) are mainly caused by abnormal adipose tissue size, distribution and metabolism and so ADSCs and their secretory factors such as EVs are currently investigated as therapeutics in these diseases. Here we provide a comprehensive summary of the current knowledge on EVs secreted from ADSCs both as diagnostic biomarkers and therapeutics in diabetes and associated cardiovascular disease, the molecular mechanisms involved, as well as on the use of ADSC differentiation potential in cardiovascular tissue repair and prostheses.
- adipose tissue-derived stem cells
- extracellular vesicles
- diabetes
- cardiovascular disease
1. Introduction
The adipose tissue has been considered for many years an inert storage depot for nutrients, but now many evidences show that adipose tissue has various physiological roles that include regulation of metabolism, immunity and endocrine function. The anatomical location in coordination with the dynamic changes of cellular component has a prominent effect on the biology of adipose tissue. Given its central role in regulation of energy homeostasis and because obesity-related disorders such as diabetes, metabolic syndrome and cardiovascular diseases have reached an epidemic magnitude, extensive interest has been payed to establish a broad map of adipose tissue cellular composition and the intercellular communication that mediate pathologic responses.
The adipose tissue is generally divided into two distinct types, white adipose tissue (WAT) and brown adipose tissue (BAT), the first acting to store and mobilize triglycerides, and the second having the leading function of burning fatty acids and glucose for heat production, a process known as adaptive (non-shivering) thermogenesis [1].
WAT is found throughout the body but is mainly organized into anatomically distinct depots: subcutaneous WAT, which is found under the skin, and visceral WAT, which is located within the body cavities, surrounding the major organs. In addition to subcutaneous and visceral fat, WAT can be found in many other areas: breasts, on the neck and upper back, extremities, in the retro-orbital space, and within bone marrow. During the last years, a third form of adipose tissue has been characterized in rodents and humans. BAT is found as depots in newborns (in perivascular and peri-organ visceral areas) but also in adults (in cervical, supraclavicular, mediastinal, and suprarenal regions) [2]. Both visceral and subcutaneous WAT depots have been shown to harbor thermogenic adipocytes, and originally in subcutaneous WAT they were called beige, whilst in visceral WAT they were termed brite [3]. The process of browning and the recruitment of beige adipocytes typically occurs in response to certain stimuli, particularly exposure to cold or to β3—adrenergic stimulation [4]. Beige adipocytes are located within WAT depots, and are morphologically and functionally comparable to brown adipocytes [5]. Beige adipocytes express uncoupling protein 1 (UCP-1), a master regulator of thermogenesis in BAT [6].
WAT is composed of a mixed population of cells including lipid-filled mature adipocytes, and a stromal vascular fraction (SVF) that contains stromal cells (adipose stem cells, blood lineage cells, vascular cells, fibroblasts) and immune cells, the percentage of each being in a dynamic state. Healthy WAT is characterized by the presence of numerous and smaller adipocytes resulted from differentiated progenitor cells. However, it is known that WAT is highly plastic and responds to different stimuli by continuous remodeling, i.e., during periods of excess energy intake it expands by both hypertrophy (increase in cell size) and/or hyperplasia (increase in cell number) [7][8][9]. The explanation for these processes comes from the fact that within WAT there are subpopulations of adipocyte progenitor cells which exhibit different secretory pro-inflammatory or fibrotic phenotypes influencing their differentiation state. Thus, identifying different subpopulations of progenitor cells and their interaction with non-stromal resident cells in WAT it is a crucial issue in order to understand how fat homeostasis is regulated.
It has been showed that expansion of visceral WAT is mainly due to adipocyte hypertrophy that is linked to inflammation and insulin resistance [10][11]. In murine models, high fat feeding leads to adipocyte hypertrophy in both visceral and subcutaneous WAT, whereas adipogenesis is thought to occur primarily in visceral WAT [7][8]. The ability of adipose tissue to expand by hyperplasia confers a protective advantage against insulin sensitivity and metabolic disease risk by the recruitment of adipocyte progenitor cells and commitment to the adipose lineage [12]. Chronic overnutrition forces adipocytes to enlarge, to store more triglycerides and when their buffering capacity is exceeded, hypertrophied adipocyte death coupled with the accumulation of pro-inflammatory macrophages and fibrotic cells into the adipose tissue leads to ectopic fat accumulation in muscle and liver and to systemic insulin resistance [13][14].
Differences between visceral and subcutaneous adipose tissue exist also in terms of the number of isolated adipose tissue-derived stem cells (ADSCs) and their ability to expand in vitro. SFV cell number isolated from omental adipose tissue was significantly higher than that from subcutaneous adipose tissue in human donors [15]. In addition, it was noted that the yield of ADSCs isolated from each gram of visceral fat was significantly greater than that from subcutaneous fat, implying that the visceral adipose tissue contained more ADSCs [16].
Extracellular vesicles (EVs) are different types of submicron vesicles derived from nearly all cells in response to cell activation, stress or apoptosis. Based on the size, morphology, and mechanism of biogenesis, they are divided in exosomes and ectosomes. Exosomes (50–100 nm) are small vesicles exocyted from multivesicular bodies (MVBs) after receptor-mediated endocytosis. Ectosomes (microvesicles (MVs) or microparticls (MPs)) are slightly larger vesicles (100–500 nm) compared with exosomes and are also cell specific as they are released from plasma membrane by budding [17].
Metabolic syndrome is a multifactorial disease and involves numerous cell types, tissues, organs and humoral factors (cytokines, growth factors and miRNA molecules, many of them being encapsulated in circulating EVs). Almost any type of cells (endothelial cells, lymphocytes, T cells, macrophages, renal cells, cancer cells and stem cells) can release EVs in physiological state, and number of these submicron vesicular membrane structures is increased during pathological processes. EVs can exhibit characteristics (RNAs, DNA and lipids) of their parent cell of origin and may provide diagnostic and prognostic value in metabolic dysfunction and cardiovascular diseases [18][19][20]. EVs can be isolated from plasma, urine, cerebral spinal fluid, lymph and conditioned media from cell culture by different methods.
Mesenchymal stem cells (MSCs) are multipotent cells with high proliferative, self-renewal, multi-lineage differentiation, and regenerative potential. MSCs reside in variable amount in adult organs of human bodies with high regeneration and differentiation capacity, but bone marrow, human umbilical cord and adipose tissue are important origins of MSCs for researches. MSCs from these sources exhibit common features in terms of proliferation and differentiation capacity, but their regenerative potential is site-specific. Previous studies [21][22] showed that MSCs convey their reparative effects by releasing EVs, including small and large EVs. Recently, MSC-derived EVs as a cell-free therapeutic alternative have gained considerable interest. More than that, EVs can also be used as a vehicle to deliver bioactive factors. EVs derived from bone marrow mesenchymal stem cells (BM-MSCs) have been used to treat cartilage defects or osteoarthritis which are related to bone diseases [23][24], renal injury [25] or Graft Versus-Host Disease [26]. Small EVs derived from BM-MSCs are widely used in the treatment of myocardial infarction (MI). An important mechanism behind the regenerative potential of EV-MSCs is the immune-regulatory properties of these vesicles. In a recent paper, small EVs secreted by BM-MSCs were incorporated into alginate hydrogel to increase their retention in the heart, have been showed to reduce the apoptosis of cardiomyocytes and promoted the polarization of macrophages [27].
Among MSC-dervied EVs, ADSC-derived EVs stand out as novel mediators and biomarkers in the crosstalk between adipose tissue and other organs/tissues relevant in obesity and metabolic diseases. In recent years, EVs from ADSCs have attracted much attention for their role in metabolic dysfunction, in particular, obesity and its complications, but their role in diabetes and associated cardiovascular disease was very little discussed.
Diabetic nephropathy represents one of the most relevant chronic complications of diabetes and the major cause of end-stage renal failure [28]. At present, available clinical biomarkers including glomerular filtration rate (GFR), proteinuria and urinary sediment evaluation do not allow a specific diagnosis neither clarify disease staging. Therefore, the finding of non-invasive biomarkers could hinder the use of kidney biopsy, a procedure implying complication risks. Urine is an ideal source of biomarkers, particularly for diseases of the kidney and urinary tract, because it can be conveniently collected in large amounts without risk to the patient. Recent studies revealed that expression of urinary exosomal miRNA is changed in patients with type 2 diabetes mellitus (T2DM) [29][30]. Urinary exosomal miRNA content is altered in patients suffering from type 1 diabetes mellitus (T1DM) with incipient diabetic nephropathy and micro-albuminuria resulting in an up-regulation of miR-130a and miR-145 and a down-regulation of miR-155 and miR-424 [31].
2. Extracellular Vesicles from Adipose Tissue Stem Cells
2.1. Classification and Molecular Properties of Adipose Tissue Extracellular Vesicles
There is no consensus on specific markers of EV subtypes, such as endosomal origin exosomes (small EVs) and plasma membrane-derived ectosomes (microparticles/large EVs), therefore assigning EVs to a particular biogenesis pathway remains very difficult. Due to an increased interest in the EVs field and of higher number of published papers working with these EV subtypes, whose size and amount often make them difficult to obtain as relatively pure preparations, and to characterize properly, an improved guideline have been published in order to help scientists to make strong conclusions on the involvement of specific populations of EVs in physiological or pathological condition. A list of minimal information for studies of EVs (MISEV2018) was provided, covering EV separation/isolation, characterization, and functional studies. This updated ISEV statement reflects an improved understanding in EV biology, which has resulted in a consensus to promote meaningful changes to nomenclature and experimental approach. MISEV2018 guidelines advise the authors to use operational terms for EV subtypes that refer to (a) physical characteristics of EVs, such as size (small EVs (sEVs) and medium/large EVs (m/lEVs), with ranges defined, for instance, respectively, <100 nm or <200 nm [small], or >200 nm [large and/or medium]) or density (low, middle, high, with each range defined); (b) biochemical composition (CD63+/CD81+-EVs, Annexin A5-stained EVs, etc.); or c) descriptions of conditions or cell of origin (podocyte EVs, hypoxic EVs, large oncosomes, apoptotic bodies) [32].
Considering the fat type and location, adipose tissue-derived EVs can be divided into: EVs secreted from subcutaneous or visceral fat and EVs secreted from WAT or BAT.
Molecular properties of adipose tissue EVs rest on their composition in lipids, proteins, and nucleic acids. Basically, the biomolecular content of EVs is similar to that of the source cell, any differences in adipose tissue-derived EVs composition resting on the content variability of the original fat cell type.
Adipose tissue EV release has been widely studied in explants from both adipose visceral and subcutaneous tissues [33][34], as well as in in vitro differentiated adipocytes and in adipose tissue stem cells (ADSCs) [33]. However, most of the in vitro studies used the murine 3T3-L1 pre-adipocyte cell line differentiated to mature adipocytes and only a few used human adipocytes and adipose tissue extracts [35].
Analyses of microRNA (miRNA) profiles, showed that adipocyte-derived small EVs exhibit abundant miRNA content, many of which are up-regulated, such as miR-103, miR-146b, miR-148a [36][37][38]. Considering the length of adipogenesis induction, 3T3-L1 small EVs, registered a time dependent increment of adipogenesis-related gene transcripts expression, peroxisome proliferator-activated receptor γ2 (PPARγ2), adiponectin and leptin [39]. Therefore, EVs from adipogenic induced 3T3-L1 cells, doubled adiponectin content, while fatty acid-binding protein 4 (FABP-4) and preadipocyte factor 1 (PREF-1) levels decreased [35]. Adiponectin, as well as a small amount of resistin were found also in small EVs from serum [40]. After adipogenesis, the lipid composition of EVs was also enriched in phosphatidiyl serine and arahidonic acid and in long chain omega-3 fatty acid decosahexaenoic acid [41]. Protein marker expression of CD9, CD36, TSG101 and Alix remained unchanged, after 3T3-L1 cell differentiation [35]. In normoxic and hypoxic conditions, 231 proteins were identified in 3T3-L1 small EVs, including enzymes involved in “de novo” lipogenesis, mostly when the hypoxic environment induced by adipocyte hypertrophy was mimicked [42]. Moreover, besides adipocyte specific proteins, several immunomodulatory proteins, such as tumor necrosis alpha (TNF-α), macrophage-colony-stimulating factor (MCSF) and retinol binding protein4 (RBP-4), have been reported within the small EVs [33].
Human adipose tissue isolated ADSCs release small EVs that have been shown to contain small RNA species like miRNAs, small nucleolar RNAs (snoRNAs) and mostly transfer RNAs (tRNAs) [43]. Protein secretory profile of ADSCs is considered almost specific to each person, thus forming a heterogeneous population of cells that may produce equivalent EVs [44].
Differentiated human adipocytes-derived EV content is characterized by adipose specific markers FABP-4 as well as adiponectin and by a number of inflammatory adipokines, including macrophage migration inhibitory factor (MIF), TNFα, MCSF, and RBP-4 [33]. Regarding the adipokine profile, visceral adipose tissue EVs have a significantly higher concentration of interleukin -6 (IL-6), MIF, and monocyte chemoattractant protein-1 (MCP-1) compared to those from subcutaneous adipose tissue. The content of EVs produced by both subcutaneous and visceral adipose tissue is also rich in adiponectin. In contrast to adiponectin-negative EVs produced mainly by stromal cells, adiponectin-positive EVs are produced exclusively by adipocytes.
Since the release of small EVs by BAT is increased after cAMP treatment, down regulation of specific marker miR-92a was also observed in both murine and human small EVs after cold exposure-dependent cAMP activation (Chen et al., 2016).
2.2. Physiological Functions of Adipose Tissue Extracellular Vesicles
Adipocyte released EVs influence the vascular health of the adipose tissue, being important means of vascular homeostasis regulation by neovascularization and angiogenesis [35], ADSC small EVs promoting vascular endothelial cell migration and proliferation and stimulating neo-vessel formation [45][46].
Adipocyte-derived EVs (including large EVs and small EVs) may function as adipokines contributing to adipose tissue homeostasis or dysfunction. An important role of EVs isolated from adipose tissue is their capacity to mediate the endocrine connection between maternal adipose tissue and fetal growth, being unfortunately also responsible for fetal overgrowth [47]. Since adipocyte-derived EVs contain large amount of adiponectin, a crucial adipokine for glucose and lipid metabolism, also involved in fatty acid oxidation and insulin sensitivity [48], small EVs through their rich adiponectin content may be involved in distant cell metabolism.
Adipocyte derived EVs have also an important role in paracrine regulation of adipocyte metabolism [35]. Microvesicles and exosomes released by adipocytes containing glycosylphosphatidylinositol (GPI)-anchored proteins, CD73 and Gcel have role in esterification and in lipolysis inhibition[49] the small EVs may be effective players in adipocyte intercommunication[50].
The paracrine cross talk between adipocytes and macrophages is also regulated by adipose tissue-derived EVs. When primary monocytes differentiate into macrophages, the most effective EVs were adiponectin-positive and visceral ones compared to adiponectin-negative and subcutaneous EVs [33]. Moreover, the exposure of hepatocytes to subcutaneous and visceral adipose tissue-derived EVs led to a negative association between Akt signaling and glucose-6-phosphatase gene expression [33]. There is evidence of adipocyte-derived EV communication with the immune cells as well as their influence on whole body. EVs are implicated in regulation of hepatic insulin signalling and immunity as shown in vitro by their ability to reduce the proliferation rate of stimulated T lymphocytes and by controlling monocyte to macrophage differentiation.
Figure 1. Relevance of extracellular vesicles from adipose tissue stem cells in pathophysiology of diabetes and associated cardiovascular disease: extracellular vesicles as diagnostic biomarkers, direct mediators and therapeutic strategies
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249598
References
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat Rev Mol Cell Biol 2016, 17, 691–702, doi:10.1038/nrm.2016.96.
- Silva, F.J.; Holt, D.J.; Vargas, V.; Yockman, J.; Boudina, S.; Atkinson, D.; Grainger, D.W.; Revelo, M.P.; Sherman, W.; Bull, D.A., et al. Metabolically active human brown adipose tissue derived stem cells. Stem cells 2014, 32, 572–581, doi:10.1002/stem.1595.
- Wang, S.; Yang, X. Inter-organ regulation of adipose tissue browning. Cell Mol Life Sci 2017, 74, 1765–1776, doi:10.1007/s00018-016-2420-x.
- Rosenwald, M.; Perdikari, A.; Rulicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013, 15, 659–667, doi:10.1038/ncb2740.
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010, 285, 7153–7164, doi:10.1074/jbc.M109.053942.
- Wu, J.; Jun, H.; McDermott, J.R. Formation and activation of thermogenic fat. Trends Genet 2015, 31, 232–238, doi:10.1016/j.tig.2015.03.003.
- Jeffery, E.; Church, C.D.; Holtrup, B.; Colman, L.; Rodeheffer, M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015, 17, 376–385, doi:10.1038/ncb3122.
- van Beek, L.; van Klinken, J.B.; Pronk, A.C.; van Dam, A.D.; Dirven, E.; Rensen, P.C.; Koning, F.; Willems van Dijk, K.; van Harmelen, V. The limited storage capacity of gonadal adipose tissue directs the development of metabolic disorders in male C57Bl/6J mice. Diabetologia 2015, 58, 1601–1609, doi:10.1007/s00125-015-3594-8.
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013, 19, 1338–1344, doi:10.1038/nm.3324.
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Ryden, M.; Frisen, J.; Bernard, S.; Arner, P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109, doi:10.2337/db09-0942.
- Lessard, J.; Laforest, S.; Pelletier, M.; Leboeuf, M.; Blackburn, L.; Tchernof, A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte 2014, 3, 197–205, doi:10.4161/adip.29385.
- Shao, M.; Vishvanath, L.; Busbuso, N.C.; Hepler, C.; Shan, B.; Sharma, A.X.; Chen, S.; Yu, X.; An, Y.A.; Zhu, Y., et al. De novo adipocyte differentiation from Pdgfrbeta(+) preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun 2018, 9, 890, doi:10.1038/s41467-018-03196-x.
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci 2019, 20, doi:10.3390/ijms20092358.
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: common threads and missing links. Cell 2012, 148, 852–871, doi:10.1016/j.cell.2012.02.017.
- Russo, V.; Yu, C.; Belliveau, P.; Hamilton, A.; Flynn, L.E. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem cells translational medicine 2014, 3, 206–217, doi:10.5966/sctm.2013-0125.
- Chi, C.; Wang, F.; Xiang, B.; Deng, J.; Liu, S.; Lin, H.Y.; Natarajan, K.; Li, G.; Wang, L.; Wang, J., et al. Adipose-derived stem cells from both visceral and subcutaneous fat deposits significantly improve contractile function of infarcted rat hearts. Cell transplantation 2015, 24, 2337–2351, doi:10.3727/096368914X685780.
- Gherghiceanu, M.; Alexandru, N.; Magda, S.L.; Constantin, A.; Nemecz, M.; Filippi, A.; Ioghen, O.C.; Ceafalan, L.; Bojin, F.; Tanko, G., et al. Part One: Extracellular Vesicles as Valuable Players in Diabetic Cardiovascular Diseases. In Extracellular Vesicles and Their Importance in Human Health, de Bona, A.G., Ed. IntechOpen: 2019.
- Hulsmans, M.; Holvoet, P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovascular research 2013, 100, 7–18, doi:10.1093/cvr/cvt161.
- Martinez, M.C.; Andriantsitohaina, R. Extracellular Vesicles in Metabolic Syndrome. Circulation research 2017, 120, 1674–1686, doi:10.1161/CIRCRESAHA.117.309419.
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A., et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388, doi:10.2337/db17-1308.
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative medicine 2011, 6, 481–492, doi:10.2217/rme.11.35.
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Molecular therapy : the journal of the American Society of Gene Therapy 2018, 26, 1610–1623, doi:10.1016/j.ymthe.2018.05.009.
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Current opinion in pharmacology 2018, 40, 74–80, doi:10.1016/j.coph.2018.03.009.
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920, doi:10.7150/thno.20746.
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Scientific reports 2019, 9, 4468, doi:10.1038/s41598-019-41100-9.
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem cells 2018, 36, 434–445, doi:10.1002/stem.2759.
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C., et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 2019, 9, 7403–7416, doi:10.7150/thno.32637.
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: global dimension and perspectives. Lancet 2013, 382, 260–272, doi:10.1016/S0140-6736(13)60687-X.
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C., et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PloS one 2013, 8, e73798, doi:10.1371/journal.pone.0073798.
- Delic, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PloS one 2016, 11, e0150154, doi:10.1371/journal.pone.0150154.
- Ramezani, A.; Devaney, J.M.; Cohen, S.; Wing, M.R.; Scott, R.; Knoblach, S.; Singhal, R.; Howard, L.; Kopp, J.B.; Raj, D.S. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. European journal of clinical investigation 2015, 45, 394–404, doi:10.1111/eci.12420.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles 2018, 7, 1535750, doi:10.1080/20013078.2018.1535750.
- Kranendonk, M.E.; Visseren, F.L.; van Balkom, B.W.; Nolte-'t Hoen, E.N.; van Herwaarden, J.A.; de Jager, W.; Schipper, H.S.; Brenkman, A.B.; Verhaar, M.C.; Wauben, M.H., et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 2014, 22, 1296–1308, doi:10.1002/oby.20679.
- Koeck, E.S.; Iordanskaia, T.; Sevilla, S.; Ferrante, S.C.; Hubal, M.J.; Freishtat, R.J.; Nadler, E.P. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity-related liver disease. J Surg Res 2014, 192, 268–275, doi:10.1016/j.jss.2014.06.050.
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. Journal of extracellular vesicles 2015, 4, 29159, doi:10.3402/jev.v4.29159.
- Chen, L.; Dai, Y.M.; Ji, C.B.; Yang, L.; Shi, C.M.; Xu, G.F.; Pang, L.X.; Huang, F.Y.; Zhang, C.M.; Guo, X.R. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol Cell Endocrinol 2014, 393, 65–74, doi:10.1016/j.mce.2014.05.022.
- Li, M.; Liu, Z.; Zhang, Z.; Liu, G.; Sun, S.; Sun, C. miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biological Chemistry 2015, 396, 235, doi:https://doi.org/10.1515/hsz-2014-0241.
- Londono Gentile, T.; Lu, C.; Lodato, P.M.; Tse, S.; Olejniczak, S.H.; Witze, E.S.; Thompson, C.B.; Wellen, K.E. DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation. Mol Cell Biol 2013, 33, 3864–3878, doi:10.1128/MCB.01495-12.
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun 2010, 398, 723–729, doi:10.1016/j.bbrc.2010.07.008.
- Phoonsawat, W.; Aoki-Yoshida, A.; Tsuruta, T.; Sonoyama, K. Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun 2014, 448, 261–266, doi:10.1016/j.bbrc.2014.04.114.
- DeClercq, V.; d'Eon, B.; McLeod, R.S. Fatty acids increase adiponectin secretion through both classical and exosome pathways. Biochim Biophys Acta 2015, 1851, 1123–1133, doi:10.1016/j.bbalip.2015.04.005.
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H., et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun 2014, 445, 327–333, doi:10.1016/j.bbrc.2014.01.183.
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N., et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem cell research & therapy 2015, 6, 127, doi:10.1186/s13287-015-0116-z.
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D., et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem cell research & therapy 2015, 6, 221, doi:10.1186/s13287-015-0209-8.
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell communication and signaling : CCS 2014, 12, 26, doi:10.1186/1478-811X-12-26.
- Pascucci, L.; Alessandri, G.; Dall'Aglio, C.; Mercati, F.; Coliolo, P.; Bazzucchi, C.; Dante, S.; Petrini, S.; Curina, G.; Ceccarelli, P. Membrane vesicles mediate pro-angiogenic activity of equine adipose-derived mesenchymal stromal cells. Vet J 2014, 202, 361–366, doi:10.1016/j.tvjl.2014.08.021.
- Jayabalan, N.; Lai, A.; Ormazabal, V.; Adam, S.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Lim, R.; Jansson, T.; McIntyre, H.D., et al. Adipose Tissue Exosomal Proteomic Profile Reveals a Role on Placenta Glucose Metabolism in Gestational Diabetes Mellitus. J Clin Endocrinol Metab 2019, 104, 1735–1752, doi:10.1210/jc.2018-01599.
- Barnea, M.; Chapnik, N.; Genzer, Y.; Froy, O. The circadian clock machinery controls adiponectin expression. Mol Cell Endocrinol 2015, 399, 284–287, doi:10.1016/j.mce.2014.10.018.
- McLaughlin, T.; Sherman, A.; Tsao, P.; Gonzalez, O.; Yee, G.; Lamendola, C.; Reaven, G.M.; Cushman, S.W. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007, 50, 1707–1715, doi:10.1007/s00125-007-0708-y.
- Muller, G. Let's shift lipid burden--from large to small adipocytes. Eur J Pharmacol 2011, 656, 1–4, doi:10.1016/j.ejphar.2011.01.035.