Being one of the most abundant proteins in human and other mammals, albumin plays a crucial role in transporting various endogenous and exogenous molecules and maintaining of colloid osmotic pressure of the blood. It is not only the passive but also the active participant of the pharmacokinetic and toxicokinetic processes possessing a number of enzymatic activities. A free thiol group of the albumin molecule determines the participation of the protein in redox reactions. Its activity is not limited to interaction with other molecules entering the blood: of great physiological importance is its interaction with the cells of blood, blood vessels and also outside the vascular bed. This topic review contains data on the enzymatic, inflammatory and antioxidant properties of serum albumin.
- albumin
- blood plasma
- enzymatic activities
- oxidative stress
1. Introduction: Physico-Chemical, Evolutionary and Genetic Aspects
Albumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water-soluble and moderately soluble in concentrated salt solutions. The key qualities of albumin are those of an acidic, highly soluble and very stable protein, able to withstand temperatures of 60 °C for 10 h [1]. Human serum albumin (HSA) has a total of 83 positively charged residues (Arg + Lys) and 98 negatively charged residues (Asp + Glu), with a theoretical pI of 5.12. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Several other blood transport proteins are evolutionarily related to serum albumin, including alpha-fetoprotein, vitamin D-binding protein and afamin [2][3]. This family is only found in vertebrates [4]. The four canonical human albumins are arranged on chromosome 4 region 4q13.3 in a tandem manner [5]. The human albumin gene is 16,961 nucleotides long from the putative ‘cap’ site to the first poly(A) addition site. It is split into 15 exons that are symmetrically placed within the three domains thought to have arisen by triplication of a single primordial domain.
Humans are not the only organisms for which serum albumin plays a critical role; albumin has also been characterised in an extensive number of species, including (but not limited to) canines, chickens, several species of frogs, lampreys, pigs and salamanders (an exhaustive list can be found at albumin.org [6]). Albumin-like proteins, which were sequenced from a sea urchin, were found to have a cysteine binding pattern similar to that seen amongst other proteins in the albumin family [7].
The precursor of serum albumin (preproalbumin) has the N-terminal peptide, which is cut off before the protein leaves the rough endoplasmic reticulum. The product of this removal (proalbumin) is transported to the Golgi apparatus. In secretory granules, the limited proteolysis occurs and the mature non-glycosylated albumin is secreted into the extracellular environment [1]. Synthesis occurs in the polysomes of hepatocytes, and in healthy adults, 10–15 g/day of albumin can be produced; this accounts for nearly 10% of total protein synthesis in the liver [8]. While 30% of albumin is maintained in the plasma, the remaining pool is found predominantly in skin and muscle tissue.
The molecule of HSA consists of 585 amino acids forming one polypeptide chain. The length of the primary sequence can be different in albumins of other species; for example, 584 amino acids in bovine serum albumin (BSA) and 583 residues in rat serum albumin (RSA). The amino acid compositions of HSA and RSA are 73.0% identical, of BSA and RSA—69.9%. Molecular weight of HSA based on amino acid composition is 66.439 kDa, BSA—66.267 kDa, RSA—65.871 kDa; however, the values of molecular weight can vary because of post-translational modifications and genetic variants. The secondary structure of the protein contains about 67% helical structures next to 33% of turn and extended chain configurations without any β-sheets [9] (Figure 1). The three-dimensional structure of HSA was resolved rather late, only in the 1990s [10]. A similar structure of BSA was obtained in 2012 [11], but the three-dimensional structure of albumin of rats, the principal animals used in pharmacological and toxicological experiments, has not been obtained yet. Three homologous domains (I, II, III), consisting of two subdomains (A, B) form a three-dimensional structure of the protein, which is rather labile (Figure 1).
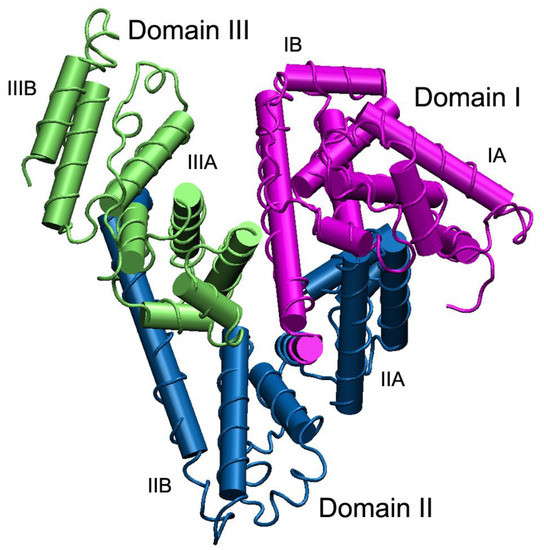
Figure 1. The structure of serum albumin. Domains I, II and III are shown in purple, blue and green, respectively; each domain consists of two subdomains A and B. The albumin molecule does not contain β-sheets, α-helices are presented as cylinders. To create the figure, a three-dimensional structure of human serum albumin from the PDB database, code 3JQZ [12], was used.
There are dozens of genetic variants of HSA (for exhaustive list see [6]). Possible effects of some single point mutations on the ligand-binding capabilities of HSA were investigated by studying the interactions between the strongly bound drugs warfarin, salicylate and diazepam, and five structurally characterised genetic variants of the protein [13]. Equilibrium dialysis data revealed pronounced reductions in high affinity binding of all three ligands to HSA Canterbury (313 Lys→Asn) and to HSA Parklands (365 Asp→His). By contrast, unchanged binding of the drugs was found in the case of HSA Verona (570 Glu→Lys). Salicylate was the only drug bound with a lower affinity to HSA Niigata (269 Asp→Gly), whereas binding of both salicylate and diazepam to HSA Roma (321 Glu→Lys) were moderately reduced. In about half of the cases of diminished binding, the primary association constant was reduced by 1 order of magnitude, giving rise to an increase in the unbound fraction of the drugs of 500% or more at therapeutically relevant molar ratios of drug and protein. Changes in protein charge play minor importance for reduced binding, though conformational changes in the 313–365 region of the proteins seem to be the main cause for diminished binding of these ligands [13].
Albumin normally is not covered with hydrocarbons and can bind different endogenous and exogenous ligands: water and metal cations, fatty acids, hormones, bilirubin, transferrin, nitric oxide, aspirin, warfarin, ibuprofen, phenylbutazone, etc. [14]. Ligand binding occurs at two primary sites (Sudlow site I in subdomain IIA and Sudlow site II in subdomain IIIA) and several secondary ones. When albumin interacts with different substances, the effects of cooperativity and allosteric modulation occurs, which is usually inherent to multimeric macromolecules [15][16]. The albumin molecule contains 17 disulfide bonds and one free thiol group in Cys34, which determines the participation of albumin in redox reactions.
2. Enzymatic Properties
Albumin is not only the passive but also the active participant of the pharmacokinetic and toxicokinetic processes. Numerous experiments showed the esterase or pseudoesterase activity of albumin against α-naphtylacetate and p-nitrophenylacetate (NPA), fatty acid esters, aspirin, ketoprofene glucuronide, cyclophosphamide, nicotinic acid esters, octanoyl ghrelin, nitroacetanilide, nitrofluoroacetanilide and organophosphorus pesticides [17][18][19]. Acetylation is a typical example of the pseudoesterase activity of albumin when the consumption of the substrate is due to the formation of covalent bonds with the participation of many amino acid residues of the albumin molecule. Acetylation of albumin by NPA was found to occur at 82 amino acids (aa) including lysine (59 aa), serine (10 aa), threonine (8 aa), tyrosine (4 aa) and aspartate (1 aa) residues [20], with adducts at the lysine residues being the most stable.
Of special interest is the phosphatase activity of albumin, i.e., the phosphomonoesterase (EC 3.1.3…?) [21], RNA-hydrolase or phosphodiesterase (EC 3.1.4.16 ?) [22] and phosphotriesterase (EC 3.1.8.1 and 3.1.8.2) [23][24] activities. The subclass 3.1.8 (hydrolases of phosphotriesters) contains aryldialkylphosphatase (EC 3.1.8.1) and diisopropylfluorophosphatase (EC 3.1.8.2) [25][26]. Aryldialkylphosphatase is known as paraoxonase, which hydrolyses esters of tribasic phosphoric acid, of dibasic phosphonic acid and of monobasic phosphinic acid. Divalent cations (mainly Ca2+) are required for activity of paraoxonase [27]; the fundamental difference of albumin is the lack of dependence on Ca2+, that is used for the differential analysis of the activities of these enzymes [24][28]. In toxicology, understanding the mechanistic interactions of organophosphates (OPs) with albumin could help in the development of new types of antidotes [29]. Among the other activities of serum albumin, one should note its prostaglandin D synthase and other activities associated with prostanoid metabolism [30][31][32]. Quite exotic activities for albumin are glucuronidase activity [33][34] and the enolase activity [35]; the latter can be used for the differential diagnostics of benign and malignant tumors.
Bovine and human serum albumins catalyse the aldol reaction of aromatic aldehydes and acetone, with saturation kinetics and moderate and opposite enantioselectivity; the reaction occurs at the binding site in domain IIa and is inhibited by warfarin [36]. A 101-amino-acid polypeptide derived from the sequence of the IIA binding site of HSA was identified, containing eight cysteine residues to form disulfide bridges that stabilise the polypeptide structure [37]. This protein retains the IIA fragment’s capacity to bind typical ligands such as warfarin and efavirenz and other albumin’s functional properties such as aldolase activity and the ability to direct the stereochemical outcome of a diketone reduction. It was suggested that some simple reactions that were catalysed by the serum albumin with Michaelis-Menten kinetics involve nonspecific substrate binding and catalysis by local functional groups [38]. These different active sites can bind promiscuously an array of hydrophobic negatively charged ligands, with a lysine residue acting as a primitive active site allowing these promiscuous activities to take place [39]. A method for predicting catalytic and substrate promiscuity using a graph-based representation known as molecular signature was suggested and enolase activity was among the first promiscuous activities described [40]. However, the binding mechanism via hydrophobic interaction with the binding site can be entirely different in presence of protein denaturing agent like urea, with electrostatic interaction playing a major role [41].
Most, if not all, enzymes are capable of catalysing physiologically irrelevant secondary promiscuous reactions in addition to the reactions that they have evolved to catalyse, and the universe of promiscuous activities available in nature was found to be enormous [42][43]. Nevertheless, we suggest that the promiscuity of albumin is principally different from that of the specialised enzymes, in that it was evolved as a result of not acquiring but a loss of some specialised activities, such as esterase (hydrolase) activities with digestive functions.
The Kemp elimination is regarded to be a prototypical reaction used to study proton abstraction from carbon. The reaction takes place at the so-called Stern layer, the interface between a micellar head or protein surface and water, and a significant rate acceleration can be achieved regardless of the precise positioning of substrates [44][45]. It is interesting to note that the reaction rate is decreased in protic solvents such as water as compared with aprotic organic solvents, the electrostatic term of the hydrogen bonds being the main factor for the large inhibitory effect of water; the presence of an external electric field oriented in the direction of the charge transfer increases the reaction rate [46]. On the other hand, the mechanism of the Kemp elimination in protein molecules was strongly associated with the presence of a catalytic base (Trp, Tyr, Phe) and a hydrogen bond donor (Lys, Arg, Ser, Tyr, His, water molecule) [47]. To this end, we were the first who explained the albumin mediated hydrolysis of some substrates by existence of catalytic dyads (as compared with catalytic triads in cholinesterases) His-Tyr or Lys-Tyr, where the histidine or lysine residues function as the acidic residues and hydrogen bond donors, whereas Tyr residue is a catalytic base [17].
In 1986, concern was expressed over the fact that the current classification of esterases did not reflect the real state of things. Albumin was just used as an example of the protein that exhibits the esterase activity but has no place in the classification [48]. The broad substrate specificity and no dependence on Ca2+ do not allow for the identification of albumin as any of the enzymes with their numbers in the enzyme nomenclature. The place of albumin in the nomenclature of enzymes remains yet to be determined.
3. Effect of Molecular Crowding on the Functional Properties of Albumin
In live systems, in contrast to in vitro experiments, biochemical processes take place in an environment containing high concentrations of macromolecules (50–400 mg/mL). Such conditions are called molecular crowding. Due to the dense environment, the volume of available solvent decreases, which might affect protein structure, folding, shape, conformational stability, binding of small molecules, enzymatic activity, protein-protein interactions, protein-nucleic acid interactions and pathological aggregation [49]. In blood plasma, the density of macromolecules reaches 80 mg/mL, which causes noticeable crowding effects and affects the conformation and functional characteristics of plasma proteins. Crowding effects have been proved for albumin too.
Ota and Takano, with the help of by Raman spectroscopy, showed that crowded milieu influenced the strength of intramolecular hydrogen bonds of BSA molecule. This effect, in turn, led to the BSA molecule adopting a more compact structure [50].
Zhu et al. studied the effect of molecular crowding on the binding of saturated medium-chain FAs and unsaturated long-chain FAs to BSA [51]. Polyethylene glycol PEG2000 was used to simulate the conditions of crowded milieu. Adding of the polymer to the medium improved the binding of medium-chained FAs but weakened the binding of the long-chain FAs. Having analysed the secondary structure of BSA, the authors have concluded that gradual increase of the medium density makes the albumin molecule more friable. Thus, taking into account the literature data available, it can be assumed that the constants of esterase and binding and esterase activity of albumin in the bloodstream will differ from the constants measured in the “ideal solution” of in vitro and in silico experiments. Definitely, it is necessary to develop the test systems that simulate the activity of albumin under conditions of molecular crowding.
4. Albumin and Redox Modulation
In healthy human organism, about 80% of all detected plasma thiols are those of albumin [52]. The Cys34 residue is able to neutralise hydrogen peroxide, peroxynitrite, superoxide anion and hypochlorous acid, being oxidised to sulfenic acid (HSA-SOH) [53][54]. A list of the albumin activities associated with redox modulation of blood plasma and intercellular liquid consists of the thioesterase [55][56], glutathione peroxidase and cysteine peroxidase activities, as well as the peroxidase activity towards lipid hydroperoxides [57][58][59]. The important role of two cysteine residues of albumin, Cys392 and Cys438 should be noted, which form redox active disulfide in the complex of albumin with palmitoyl-CoA [59]. Albumin is a trap of radicals due to six methionine residues, but Cys34 is the most important for this function [53][60]. The N-terminal region of human albumin, Asp-Ala-His-Lys, in the complex with cuprum ions has the superoxide dismutase activity [61]. Albumin can stoichiometrically inactivate hydrogen peroxide and peroxynitrite due to reversible oxidation of the Cys34 residue to the sulfenic acid derivative [53]. This group of activities may probably be supplemented by the cyanide detoxification reaction with the formation of thiocyanate, which is catalysed by the regions of subdomain IIIA without the involvement of Tyr411 [62]. In addition, the prooxidant properties of albumin should be noted: the albumin-bound Cu2+ ions strengthen the formation of ascorbate radical, followed by oxidation of the formed Cu+ ions by molecular oxygen and protons again to Cu2+ [63]. Albumin is more exposed to the reactive oxygen species (ROS) than other proteins, and the percentage of oxidised albumin serves as a biomarker of the severity of oxidative stress accompanying various diseases. The level of Cys34-cysteinylated albumin is significantly increased in patients suffering from diabetes mellitus, liver and kidney diseases [64]. Oxidised albumin has been proved to be a biomarker of the heaviness of such pathologies as Duchenne muscular dystrophy [65], Alzheimer’s and Parkinson’s diseases [66][67], hyperparathyroidism [68], acute ischemic stroke [69], etc. An extensive study of Japanese residents revealed that the risk of atherosclerosis is inversely correlated with the percentage of oxidised form of serum albumin [70]. In 2020, Violi et al. demonstrated that there is an independent direct correlation of mortality in COVID-19 with HSA level. The author suggested that this association might be connected with anticoagulant and antioxidant properties of albumin [71].
Glycation (covalent binding of monosaccharides to the side chains of arginines and lysines) is another possible chemical modification that can affect the structural and functional features of albumin [72]. More than 60 albumin glycation sites have been identified so far, and Lys525 is considered to be the most reactive one [73][74]. As in the case of the effect of Cys34 oxidation on the binding activity of Sudlow sites, the data on the effect of glycation on the antioxidant properties of albumin are contradictory [75][76][77][78]. The controversial behavior of glycosylated albumin in biochemical experiments might be due to interspecies differences, the nature and concentration of the involved carbohydrates (glucose, methylglyoxal), and the conditions of incubation with monosaccharides [72]. The differences between human and bovine albumin are of particular interest: glycation of HSA sharply decreases its antioxidant activity, while glycation of BSA tends to enhance its antioxidant properties. These data correlate with the results of computational experiments aimed at studying the effect of the redox status of HSA and BSA on their binding and esterase activity towards paraoxon [79][80]. According to the data, human and bovine albumins react differently to the oxidation of Cys34 to sulfenic and sulfinic acids.
Fatty acids (FAs) appear to play the main role in the regulation of the antioxidant properties of albumin. For the first time, this conclusion was made by Gryzunov and co-authors [63]. Binding of FAs changed the conformations of Sudlow sites I and II and increased the fluorescence quantum yield of the probes dansylamide (ligand of Sudlow site I) and dansylsarcosine (ligand of Sudlow site II); further, FAs increased the steric availability of Cys34 thiol group and strengthened its reactivity towards 5,5’-dithiobis-2-nitrobenzoic acid (DTNB). Thus, the binding of FAs by albumin makes possible a finely regulated conjugation of two important activities of the protein: ligand transport and antioxidant defense [63]. Additionally, albumin is able to enhance the antioxidant protection of the body by binding bilirubin (a ligand of Site III [81]) and polyunsaturated fatty acids, which interact with residues Arg117, Lys351 and Lys475 [53].
5. Albumin Interaction with Endothelial Cells Is a Basis for Its Diagnostic and Therapeutic Application
The level of albumin in blood plasma or serum, as well as in urine, from the point of view of diagnostics is the level of not just a major, but an integrative protein of the whole organism, which depends on two fundamental properties: the protein-synthesising function of the liver (hence its role as a negative acute phase protein [82]) and the functional state of the vascular endothelium, which determines the integrity of the blood-tissue barriers. The relationship between endothelial integrity and the level of albumin in urine is the most studied phenomenon in medical practice, indicating primarily the pathology of the kidneys, but also the state of other components of the blood and cardiovascular system [83][84][85].
Endothelial glycocalyx is a membrane-bound covering of the endothelial cells that is found on the intraluminal surfaces of all blood vessels and organs and is composed of glycoproteins that hold between 700 and 1000 mL of non-circulating plasma volume. This intraluminal layer maintains its own colloidal-osmotic pressure (COP) because of its plasma protein content (primarily albumin) that is trapped within the endothelial glycocalyx layer (EGL). Subsequently, it also has a higher COP than that of circulating plasma because of its retention of plasma proteins [86]. For this reason, the EGL is thought to contribute to approximately 60% of the intravascular COP [87]. Structurally, the EGL is a negatively charged gel-like layer that is composed of an intricate array of oligosaccharide and polysaccharide chains called glycosaminoglycans (e.g., heparan sulfate, hyaluronic acid, chondroitin sulfate, dermatan sulfate and keratan sulfate), which are covalently bonded to glycosylated membrane proteins called proteoglycans (antithrombin III, integrins and selectins) and membrane bound proteoglycans such as syndecans, glypicans and perlecans, and other plasma proteins. The EGL ranges in thickness between 0.1 and 4.5 µm, depending on the location/size of the vessel, and maintains an active reservoir of proteins and polysaccharides, such as antithrombin III and heparan sulfate [86]. An intact EGL maintains a separation between circulating plasma and vascular endothelial cells, creating an “exclusion zone”, which keeps the formed elements of the blood (red cells, white cells and platelets) from actually contacting the surface of the endothelial cells. In the presence of an intact EGL, water and electrolytes will pass freely across this layer and then beyond the endothelial cells through the intercellular clefts. With the exception of albumin, this exclusion zone also prevents high molecular weight colloids that are >70 kDa from contacting the endothelial cells. Albumin is the only major plasma protein that moves easily between the plasma and the EGL because of the selectively permeability nature of the EGL to natural colloids with molecular weights < 70 kDa [88]. A detailed schematic illustration of the space between the plasma and the EGL with albumin molecules moving through the cleft is shown in Figure 1b of the work of Kundra et al. [89].
A large-scale search for new diagnostic indicators using modern metabolomics technologies made it possible to identify from a huge set of only four simple indicators of blood plasma, including the level of albumin, which with high accuracy allows us to assess the severity of a person’s health condition and predict the likelihood of mortality for patients, regardless of their age, gender and nature of the disease [90]. In patients of intensive care units, the use of a simple ratio of positive (C-reactive protein) and negative (albumin) acute phase proteins can significantly increase the accuracy of the assessment of the risk of death [91]. Urine albumin/creatinine ratio is one the most sensitive indicators of glomerular renal dysfunction and hypertension in patient with high-risk neuroblastoma treated with myeloablative regimens [92].
A compromised blood-brain barrier leads to leakage of plasma components across the endothelial cell monolayer. Increased levels of K+ and Glu enhance neuron excitability. Extravasated albumin is taken up by astrocytes via TGF-βR and leads to Smad2-mediated downregulation of the K+ channel Kir4.1; decreased expression of Glu transporter EAAT-2 is initiated by astrocytic TNF-α. Both mechanisms exacerbate neuronal hyperactivity due to impaired K+ and Glu buffering by astrocytes [93].
Competent application of regression analysis methods makes it possible to increase the sensitivity and specificity of the diagnosis of diabetic complications due to the use of “internal” albumin indices, such as the ratio of its reduced and oxidised forms [94]. The rate of oxidised albumin to total albumin can be enhanced in liver, diabetes plus fatigue and coronary artery diseases, leading to bacterial/viral infections and eventually death in severe conditions. Due to the induction of cytokine storm, the level of oxidised albumin in serum of COVID-19 patients may be a positive predictor of mortality [95].
Albumin can serve not only as a biomarker of severity of different pathologies, but as a therapeutic substance, too. It was demonstrated that albumin can be used for delivering of reactive sulfur species (RSS) to melanoma cells enhancing the inhibition of melanin synthesis [96]. Schneider et al. showed that continuous infusion of 4% albumin in patients of intensive care unit (ICU) decreased the risk of nosocomial infections [97]. According to the data obtained, albumin reduces the oxidised form of vasostatin-1 and thus restored its antimicrobial properties.
The ability of albumin to bind water can be used to treat OPs poisoning. A decrease of glycocalyx leads to a decrease of oncotic pressure and to hypovolemia, so that appropriate compensation could become one of the therapeutic factors in acute poisoning with OPs in order to reduce the risk of death and prevent the delayed pathology. Indeed, there are described cases of successful use of fresh frosen plasma in the treatment of the so-called “intermediate syndrome”, one of the possible consequences of OPs poisoning [98].
6. Conclusions
Serum albumin is able to bind almost all known drugs, nutraceuticals and toxic substances. The protein largely determines their pharmaco- and toxicokinetics, transporting them to target tissues or sites of their biotransformation. Simultaneously, the albumin molecule can bind up to ten ligand molecules, and for this reason, many substances compete with each other for binding sites. In addition to direct competition, the protein is susceptible to allosteric modulation: binding of a ligand in one site can affect the efficiency of binding in another. Due to the thiol group within Cys34, albumin can serve as a trap for reactive oxygen and nitrogen species and participate in redox processes in the body. Moreover, this protein has a number of enzymatic activities: (pseudo) esterase, paraoxonase, phosphotriesterase, thioesterase, glutathione peroxidase and some others. The environment of the bloodstream can significantly affect the functional properties of albumin; the major fatty acids and molecular crowding display the most pronounced influence. This effect should be taken into account when studying the interaction of pharmaceuticals and xenobiotics with albumin in in vitro and in silico experiments. Interaction with EGL largely determines the integrative role of both albumin and endothelium. All these features suggest that a targeted influence on albumin to modulate its binding, enzymatic and antioxidant properties can become a kind of adjuvant therapy for pathological conditions.
This entry is adapted from the peer-reviewed paper 10.3390/antiox9100966
References
- Peters, T., Jr. All about albumin. In Biochemistry, Genetics, and Medical Applications; Academic Press Ltd: London, UK, 1996.
- Haefliger, D.N.; Moskaitis, J.E.; Schoenberg, D.R.; Wahli, W. Amphibian albumins as members of the albumin, alpha-fetoprotein, vitamin D-binding protein multigene family. J. Mol. Evol. 1989, 29, 344–354.
- Lichenstein, H.S.; Lyons, D.E.; Wurfel, M.M.; Johnson, D.A.; McGinley, M.D.; Leidli, J.C.; Trollinger, D.B.; Mayer, J.P.; Wright, S.D.; Zukowski, M.M. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J. Biol. Chem. 1994, 269, 18149–18154.
- Li, S.; Cao, Y.; Geng, F. Genome-Wide Identification and Comparative Analysis of Albumin Family in Vertebrates. Evol. Bioinform. Online 2017, 13, 1176934317716089.
- Nishio, H.; Heiskanen, M.; Palotie, A.; Bélanger, L.; Dugaiczyk, A. Tandem arrangement of the human serum albumin multigene family in the sub-centromeric region of 4q: Evolution and chromosomal direction of transcription. J. Mol. Biol. 1996, 259, 113–119.
- Albumin. Available online: http://albumin.org/ (accessed on 22 December 2020).
- Godin, R.E.; Urry, L.A.; Ernst, S.G. Alternative splicing of the Endo16 transcript produces differentially expressed mRNAs during sea urchin gastrulation. Dev. Biol. 1996, 179, 148–159.
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219.
- Lu, R.; Li, W.W.; Katzir, A.; Raichlin, Y.; Yu, H.Q.; Mizaikoff, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770.
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215.
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1278–1289.
- Hein, K.L.; Kragh-Hansen, U.; Morth, J.P.; Jeppesen, M.D.; Otzen, D.; Møller, J.V.; Nissen, P. Crystallographic analysis reveals a unique lidocaine binding site on human serum albumin. J. Struct. Biol. 2010, 171, 353–360.
- Kragh-Hansen, U.; Brennan, S.O.; Galliano, M.; Sugita, O. Binding of warfarin, salicylate, and diazepam to genetic variants of human serum albumin with known mutations. Mol. Pharmacol. 1990, 37, 238–242.
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796.
- Ascenzi, P.; Bocedi, A.; Notari, S.; Fanali, G.; Fesce, R.; Fasano, M. Allosteric modulation of drug binding to human serum albumin. Mini Rev. Med. Chem. 2006, 6, 483–489.
- Ascenzi, P.; Fasano, M. Allostery in a monomeric protein: The case of human serum albumin. Biophys. Chem. 2010, 148, 16–22.
- Goncharov, N.V.; Belinskaya, D.A.; Razygraev, A.V.; Ukolov, A.I. On the enzymatic activity of albumin. Russ. J. Bioorg. Chem. 2015, 41, 113–124.
- Lee, W.Q.; Affandi, I.S.; Feroz, S.R.; Mohamad, S.B.; Tayyab, S. Evaluation of pendimethalin binding to human serum albumin: Insights from spectroscopic and molecular modeling approach. J. Biochem. Mol. Toxicol. 2017, 31.
- Želonková, K.; Havadej, S.; Verebová, V.; Holečková, B.; Uličný, J.; Staničová, J. Fungicide Tebuconazole Influences the Structure of Human Serum Albumin Molecule. Molecules 2019, 24, 3190.
- Lockridge, O.; Xue, W.; Gaydess, A.; Grigoryan, H.; Ding, S.J.; Schopfer, L.M.; Hinrichs, S.H.; Masson, P. Pseudo-esterase activity of human albumin: Slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J. Biol. Chem. 2008, 283, 22582–22590.
- Kwon, C.H.; Maddison, K.; LoCastro, L.; Borch, R.F. Accelerated decomposition of 4-hydroxycyclophosphamide by human serum albumin. Cancer Res. 1987, 47, 1505–1508.
- Gerasimova, Y.V.; Bobik, T.V.; Ponomarenko, N.A.; Shakirov, M.M.; Zenkova, M.A.; Tamkovich, N.V.; Popova, T.V.; Knorre, D.G.; Godovikova, T.S. RNA-hydrolyzing activity of human serum albumin and its recombinant analogue. Bioorg. Med. Chem. Lett. 2010, 20, 1427–1431.
- Li, B.; Nachon, F.; Froment, M.T.; Verdier, L.; Debouzy, J.C.; Brasme, B.; Gillon, E.; Schopfer, L.M.; Lockridge, O.; Masson, P. Binding and hydrolysis of soman by human serum albumin. Chem. Res. Toxicol. 2008, 21, 421–431.
- Sogorb, M.A.; García-Argüelles, S.; Carrera, V.; Vilanova, E. Serum albumin is as efficient as paraxonase in the detoxication of paraoxon at toxicologically relevant concentrations. Chem. Res. Toxicol. 2008, 21, 1524–1529.
- Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). The Enzyme List, Class 3—Hydrolases. Generated from the ExplorEnz Database. September 2010. Available online: http://www.enzyme-database.org/downloads/ec3.pdf (accessed on 14 December 2020).
- Schomburg, D.; Schomburg, I. Springer Handbook of Enzymes. EC Number Index; Springer: Heidelberg/Berlin, Germany; New York, NY, USA, 2013.
- Kurdyukov, I.D.; Shmurak, V.I.; Nadeev, A.D.; Voitenko, N.G.; Prokofieva, D.S.; Goncharov, N.V. “Esterase status” of an organism at exposure by toxic substances and pharmaceuticals. Toksikol. Vestnik (Toxicol. Bull.) 2012, 6, 6–13.
- Sogorb, M.A.; Vilanova, E. Serum albumins and detoxication of anti-cholinesterase agents. Chem. Biol. Interact. 2010, 187, 325–329.
- Goncharov, N.V.; Belinskaia, M.A.; Shmurak, V.I.; Terpilowski, M.A.; Jenkins, R.O.; Avdonin, P.V. Serum Albumin Binding and Esterase Activity: Mechanistic Interactions with Organophosphates. Molecules 2017, 22, 1201.
- Fitzpatrick, F.A.; Wynalda, M.A. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 1983, 258, 11713–11718.
- Kimzey, M.J.; Yassine, H.N.; Riepel, B.M.; Tsaprailis, G.; Monks, T.J.; Lau, S.S. New site(s) of methylglyoxal-modified human serum albumin, identified by multiple reaction monitoring, alter warfarin binding and prostaglandin metabolism. Chem. Biol. Interact. 2011, 192, 122–128.
- Yamaguchi, S.; Aldini, G.; Ito, S.; Morishita, N.; Shibata, T.; Vistoli, G.; Carini, M.; Uchida, K. Delta12-prostaglandin J2 as a product and ligand of human serum albumin: Formation of an unusual covalent adduct at His146. J. Am. Chem. Soc. 2010, 132, 824–832.
- Dubois-Presle, N.; Lapicque, F.; Maurice, M.H.; Fournel-Gigleux, S.; Magdalou, J.; Abiteboul, M.; Siest, G.; Netter, P. Stereoselective esterase activity of human serum albumin toward ketoprofen glucuronide. Mol. Pharmacol. 1995, 47, 647–653.
- Georges, H.; Presle, N.; Buronfosse, T.; Fournel-Gigleux, S.; Netter, P.; Magdalou, J.; Lapicque, F. In Vitro stereoselective degradation of carprofen glucuronide by human serum albumin. Characterization of sites and reactive amino acids. Chirality 2000, 12, 53–62.
- Drmanovic, Z.; Voyatzi, S.; Kouretas, D.; Sahpazidou, D.; Papageorgiou, A.; Antonoglou, O. Albumin possesses intrinsic enolase activity towards dihydrotestosterone which can differentiate benign from malignant breast tumors. Anticancer. Res. 1999, 19, 4113–4124.
- Benedetti, F.; Berti, F.; Bidoggia, S. Aldolase activity of serum albumins. Org. Biomol. Chem. 2011, 9, 4417–4420.
- Luisi, I.; Pavan, S.; Fontanive, G.; Tossi, A.; Benedetti, F.; Savoini, A.; Maurizio, E.; Sgarra, R.; Sblattero, D.; Berti, F. An albumin-derived peptide scaffold capable of binding and catalysis. PLoS ONE 2013, 8, 56469.
- Kirby, A.J.; Hollfelder, F.; Tawfik, D.S. Nonspecific catalysis by protein surfaces. Appl. Biochem. Biotechnol. 2000, 83, 173–180.
- James, L.C.; Tawfik, D.S. Catalytic and binding poly-reactivities shared by two unrelated proteins: The potential role of promiscuity in enzyme evolution. Protein Sci. 2001, 10, 2600–2607.
- Carbonell, P.; Faulon, J.L. Molecular signatures-based prediction of enzyme promiscuity. Bioinformatics 2010, 26, 2012–2019.
- Moyon, N.S.; Islam, M.M.; Phukan, S.; Mitra, S. Fluorescence modulation and associative behavior of lumazine in hydrophobic domain of micelles and bovine serum albumin. J. Photochem. Photobiol. B 2013, 121, 37–45.
- Copley, S.D. Shining a light on enzyme promiscuity. Curr. Opin. Struct. Biol. 2017, 47, 167–175.
- Yang, G.; Miton, C.M.; Tokuriki, N. A mechanistic view of enzyme evolution. Protein Sci. 2020, 29, 1724–1747.
- Sanchez, E.; Lu, S.; Reed, C.; Schmidt, J.; Forconi, M. Kemp Elimination in Cationic Micelles: Designed Enzyme-Like Rates Achieved through the Addition of Long-Chain Bases. J. Phys. Org. Chem. 2016, 29, 185–189.
- Sakamoto, S.; Komatsu, T.; Ueno, T.; Hanaoka, K.; Urano, Y. Fluorescence detection of serum albumin with a turnover-based sensor utilizing Kemp elimination reaction. Bioorg. Med. Chem. Lett. 2017, 27, 3464–3467.
- Acosta-Silva, C.; Bertran, J.; Branchadell, V.; Oliva, A. Kemp Elimination Reaction Catalyzed by Electric Fields. Chemphyschem 2020, 21, 295–306.
- Röthlisberger, D.; Khersonsky, O.; Wollacott, A.M.; Jiang, L.; DeChancie, J.; Betker, J.; Gallaher, J.L.; Althoff, E.A.; Zanghellini, A.; Dym, O.; et al. Kemp elimination catalysts by computational enzyme design. Nature 2008, 453, 190–195.
- Pen, J.; Beintema, J.J. Nomenclature of esterases. Biochem. J. 1986, 240, 933.
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140.
- Ota, C.; Takano, K. Behavior of Bovine Serum Albumin Molecules in Molecular Crowding Environments Investigated by Raman Spectroscopy. Langmuir 2016, 32, 7372–7382.
- Zhu, T.T.; Zhang, Y.; Luo, X.A.; Wang, S.Z.; Jia, M.Q.; Chen, Z.X. Difference in Binding of Long- and Medium-Chain Fatty Acids with Serum Albumin: The Role of Macromolecular Crowding Effect. J. Agric. Food Chem. 2018, 66, 1242–1250.
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253.
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787.
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4.
- Pedersen, A.O.; Jacobsen, J. Reactivity of the thiol group in human and bovine albumin at pH 3–9, as measured by exchange with 2,2′-dithiodipyridine. Eur. J. Biochem. 1980, 106, 291–295.
- Agarwal, R.P.; Phillips, M.; McPherson, R.A.; Hensley, P. Serum albumin and the metabolism of disulfiram. Biochem. Pharmacol. 1986, 35, 3341–3347.
- Hurst, R.; Bao, Y.; Ridley, S.; Williamson, G. Phospholipid hydroperoxide cysteine peroxidase activity of human serum albumin. Biochem. J. 1999, 338, 723–728.
- Cha, M.K.; Kim, I.H. Glutathione-linked thiol peroxidase activity of human serum albumin: A possible antioxidant role of serum albumin in blood plasma. Biochem. Biophys. Res. Commun. 1996, 222, 619–625.
- Lee, H.; Kim, I.H. Thioredoxin-linked lipid hydroperoxide peroxidase activity of human serum albumin in the presence of palmitoyl coenzyme A. Free Radic. Biol. Med. 2001, 30, 327–333.
- Iwao, Y.; Ishima, Y.; Yamada, J.; Noguchi, T.; Kragh-Hansen, U.; Mera, K.; Honda, D.; Suenaga, A.; Maruyama, T.; Otagiri, M. Quantitative evaluation of the role of cysteine and methionine residues in the antioxidant activity of human serum albumin using recombinant mutants. IUBMB Life 2012, 64, 450–454.
- Bar-Or, D.; Rael, L.T.; Lau, E.P.; Rao, N.K.; Thomas, G.W.; Winkler, J.V.; Yukl, R.L.; Kingston, R.G.; Curtis, C.G. An analog of the human albumin N-terminus (Asp-Ala-His-Lys) prevents formation of copper-induced reactive oxygen species. Biochem. Biophys. Res. Commun. 2001, 284, 856–862.
- Jarabak, R.; Westley, J. Localization of the sulfur-cyanolysis site of serum albumin to subdomain 3-AB. J. Biochem. Toxicol. 1991, 6, 65–70.
- Gryzunov, Y.A.; Arroyo, A.; Vigne, J.L.; Zhao, Q.; Tyurin, V.A.; Hubel, C.A.; Gandley, R.E.; Vladimirov, Y.A.; Taylor, R.N.; Kagan, V.E. Binding of fatty acids facilitates oxidation of cysteine-34 and converts copper-albumin complexes from antioxidants to prooxidants. Arch. Biochem. Biophys. 2003, 413, 53–66.
- Nagumo, K.; Tanaka, M.; Chuang, V.T.; Setoyama, H.; Watanabe, H.; Yamada, N.; Kubota, K.; Tanaka, M.; Matsushita, K.; Yoshida, A.; et al. Cys34-cysteinylated Human Serum Albumin Is a Sensitive Plasma Marker in Oxidative Stress-Related Chronic Diseases. PLoS ONE 2014, 9, 85216.
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne muscular dystrophy: Myonecrosis, inflammation and oxidative stress. Dis. Model. Mech. 2020, 13, dmm043638.
- Costa, M.; Horrillo, R.; Ortiz, A.M.; Pérez, A.; Mestre, A.; Ruiz, A.; Boada, M.; Grancha, S. Increased Albumin Oxidation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients. J. Alzheimers Dis. 2018, 63, 1395–1404.
- Ueno, S.I.; Hatano, T.; Okuzumi, A.; Saiki, S.; Oji, Y.; Mori, A.; Koinuma, T.; Fujimaki, M.; Takeshige-Amano, H.; Kondo, A.; et al. Nonmercaptalbumin as an oxidative stress marker in Parkinson’s and PARK2 disease. Ann. Clin. Transl. Neurol. 2020, 7, 307–317.
- Nasif, W.A.; Mukhtar, M.H.; El-Emshaty, H.M.; Alwazna, A.H. Redox State of Human Serum Albumin and Inflammatory Biomarkers in Hemodialysis Patients with Secondary Hyperparathyroidism During Oral Calcitriol Supplementation for Vitamin, D. Open Med. Chem. J. 2018, 12, 98–110.
- Rael, L.T.; Leonard, J.; Salottolo, K.; Bar-Or, R.; Bartt, R.E.; Wagner, J.C.; Bar-Or, D. Plasma Oxidized Albumin in Acute Ischemic Stroke Is Associated With Better Outcomes. Front. Neurol. 2019, 10, 709.
- Fujii, R.; Ueyama, J.; Aoi, A.; Ichino, N.; Osakabe, K.; Sugimoto, K.; Suzuki, K.; Hamajima, N.; Wakai, K.; Kondo, T. Oxidized human serum albumin as a possible correlation factor for atherosclerosis in a rural Japanese population: The results of the Yakumo Study. Environ. Health. Prev. Med. 2018, 23, 1.
- Violi, F.; Cangemi, R.; Romiti, G.F.; Ceccarelli, G.; Oliva, A.; Alessandri, F.; Pirro, M.; Pignatelli, P.; Lichtner, M.; Carraro, A.; et al. Is Albumin Predictor of Mortality in COVID-19? Antioxid. Redox Signal 2020.
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658.
- Soboleva, A.; Mavropulo-Stolyarenko, G.; Karonova, T.; Thieme, D.; Hoehenwarter, W.; Ihling, C.; Stefanov, V.; Grishina, T.; Frolov, A. Multiple Glycation Sites in Blood Plasma Proteins as an Integrated Biomarker of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 2329.
- Qiu, H.; Jin, L.; Chen, J.; Shi, M.; Shi, F.; Wang, M.; Li, D.; Xu, X.; Su, X.; Yin, X.; et al. Comprehensive Glycomic Analysis Reveals That Human Serum Albumin Glycation Specifically Affects the Pharmacokinetics and Efficacy of Different Anticoagulant Drugs in Diabetes. Diabetes 2020, 69, 760–770.
- Bourdon, E.; Loreau, N.; Blache, D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999, 13, 233–244.
- Chesne, S.; Rondeau, P.; Armenta, S.; Bourdon, E. Effects of oxidative modifications induced by the glycation of bovine serum albumin on its structure and on cultured adipose cells. Biochimie 2006, 88, 1467–1477.
- Rondeau, P.; Singh, N.R.; Caillens, H.; Tallet, F.; Bourdon, E. Oxidative stresses induced by glycoxidized human or bovine serum albumin on human monocytes. Free Radic. Biol. Med. 2008, 45, 799–812.
- Martinez Fernandez, A.; Regazzoni, L.; Brioschi, M.; Gianazza, E.; Agostoni, P.; Aldini, G.; Banfi, C. Pro-oxidant and pro-inflammatory effects of glycated albumin on cardiomyocytes. Free Radic. Biol. Med. 2019, 144, 245–255.
- Belinskaia, D.A.; Terpilovskii, M.A.; Batalova, A.A.; Goncharov, N.V. Effect of Cys34 oxidation state of albumin on its interaction with paraoxon according to molecular modeling data. Russ. J. Bioorg. Chem. 2019, 45, 535–544.
- Belinskaia, D.A.; Batalova, A.A.; Goncharov, N.V. Effect of the bovine serum albumin redox state on its interaction with paraoxon as determined by molecular modeling [Article in Russian]. J. Evol. Biochem. Physiol. 2020, 56, 376–379.
- Zunszain, P.A.; Ghuman, J.; McDonagh, A.F.; Curry, S. Crystallographic analysis of human serum albumin complexed with 4Z,15E-bilirubin-IXalpha. J. Mol. Biol. 2008, 381, 394–406.
- Tsirpanlis, G.; Bagos, P.; Ioannou, D.; Bleta, A.; Marinou, I.; Lagouranis, A.; Chatzipanagiotou, S.; Nicolaou, C. Serum albumin: A late-reacting negative acute-phase protein in clinically evident inflammation in dialysis patients. Nephrol. Dial. Transplant. 2005, 20, 658–660.
- Thi, T.N.D.; Gia, B.N.; Thi, H.L.L.; Thi, T.N.C.; Thanh, H.P. Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J. Med. Biochem. 2020, 39, 224–230.
- Chen, L.; Jin, C.; Chen, L.; Li, M.; Zhong, Y.; Xu, Y. Value of microalbuminuria in the diagnosis of heart failure with preserved ejection fraction. Herz 2020. Epub ahead of print.
- Arogundade, F.A. Detection of Early Renal Disease In Children With Sickle Cell Anaemia Using Microalbuminuria As A Surrogate Marker. West. Afr. J. Med. 2020, 37, 327.
- Myers, G.J.; Wegner, J. Endothelial Glycocalyx and Cardiopulmonary Bypass. J. Extra Corpor. Technol. 2017, 49, 174–181.
- Perrin, R.M.; Harper, S.J.; Bates, D.O. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem. Biophys. 2007, 49, 65–72.
- Bruegger, D.; Rehm, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Conzen, P.; Becker, B.F. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit. Care 2008, 12, R73.
- Kundra, P.; Goswami, S. Endothelial glycocalyx: Role in body fluid homeostasis and fluid management. Indian J. Anaesth. 2019, 63, 6–14.
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.L.; Mägi, R.; et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med. 2014, 11, 1001606.
- Hwang, J.C.; Jiang, M.Y.; Lu, Y.H.; Wang, C.T. Precedent fluctuation of serum hs-CRP to albumin ratios and mortality risk of clinically stable hemodialysis patients. PLoS ONE 2015, 10, 0120266.
- Suominen, A.; Jahnukainen, T.; Ojala, T.H.; Sarkola, T.; Turanlahti, M.; Saarinen-Pihkala, U.M.; Jahnukainen, K. Long-term renal prognosis and risk for hypertension after myeloablative therapies in survivors of childhood high-risk neuroblastoma: A nationwide study. Pediatr. Blood Cancer 2020, 67, e28209.
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429.
- Fukuhara, S.; Yasukawa, K.; Sato, M.; Ikeda, H.; Inoguchi, Y.; Etoh, T.; Masakado, M.; Umeda, F.; Yatomi, Y.; Yamauchi, T.; et al. Clinical usefulness of human serum nonmercaptalbumin to mercaptalbumin ratio as a biomarker for diabetic complications and disability in activities of daily living in elderly patients with diabetes. Metabolism 2020, 103, 153995.
- Rahmani-Kukia, N.; Abbasi, A.; Pakravan, N.; Hassan, Z.M. Measurement of oxidized albumin: An opportunity for diagnoses or treatment of COVID-19. Bioorg. Chem. 2020, 105, 104429.
- Ikeda, M.; Ishima, Y.; Kinoshita, R.; Chuang, V.T.G.; Tasaka, N.; Matsuo, N.; Watanabe, H.; Shimizu, T.; Ishida, T.; Otagiri, M.; et al. A novel S-sulfhydrated human serum albumin preparation suppresses melanin synthesis. Redox Biol. 2018, 14, 354–360.
- Schneider, F.; Dureau, A.F.; Hellé, S.; Betscha, C.; Senger, B.; Cremel, G.; Boulmedais, F.; Strub, J.M.; Corti, A.; Meyer, N.; et al. A Pilot Study on Continuous Infusion of 4% Albumin in Critically Ill Patients: Impact on Nosocomial Infection via a Reduction Mechanism for Oxidized Substrates. Crit. Care Explor. 2019, 1, 0044.
- Yilmaz, M.; Sebe, A.; Ay, M.O.; Gumusay, U.; Topal, M.; Atli, M.; Icme, F.; Satar, S. Effectiveness of therapeutic plasma exchange in patients with intermediate syndrome due to organophosphate intoxication. Am. J. Emerg. Med. 2013, 31, 953–957.
