Porous carbons are an important class of porous materials that have grown rapidly in recent years. They have the advantages of a tunable pore structure, good physical and chemical stability, a variable specific surface, and the possibility of easy functionalization.
- porous materials
- activated carbon
- biomass
1. Introduction
Porous materials are defined as materials with cavities, or channels, called pores. Pores are empty spaces that exist between particles of any shape in materials. These empty spaces form the porosity of the materials, defined as the volume percentage of air in the material that correspond to the total volume occupied by the voids of the material divided by the total volume of the material [1]. The porous space is a continuity of absence of solid matter nested in the continuity of solid matter. It is essentially irregular in its forms and inconsistent also in its qualities, which can be given great complexity. There are two types of porosity: open porosity (on the external environment) and closed porosity. In all cases, it is generally characterized by its volume fraction (or by the density of the porous material) and the pore size distribution. The open porosity notably controls the adhesion properties of the coatings on the surface of the material [2].
Porous materials have attracted the attention of chemists and materials scientists, and the development of new porous materials has accelerated research development in recent decades [3][4][5]. This attention comes back not only to the commercial interest that porous materials have for their application in various fields such as separation [6][7], catalysis [7][8][9], adsorption [10][11][12][13][14][15], energy storage and conversion [16][17][18][19][20][21][22][23][24], and medicine [25][26], but also, because of the scientific interest in the challenges posed by their synthesis, their treatment and their characterization. Indeed, the progress of technology and the demand for porous materials have pushed researchers to develop synthesis methods allowing for controlling the parameters that determine the structural and textural characteristics of these materials. In this context, a lot of works have shown that controlling the pore size is essential for many specific applications. However, this technological and industrial development with the growth of the world population and domestic activities are causing a remarkable increase in water pollution. It is therefore necessary to purify wastewater before it is released into the environment. Thus, several wastewater treatment technologies have been developed, the adsorption of which on porous materials is proving to be one of the most promising techniques for the removal of pollutants from wastewater due to its affordable price and ease of disposal at large-scale use [27][28][29][30]. The most widely used adsorbents are activated carbons, and they are considered the most useful for the removal of organic and inorganic pollutants due to their structural variability (macro-, meso-, and micropores), their large specific surface area, and a wide availability of functional groups [31][32].
The purpose of this review is to discuss the preparation processes of porous carbons as well as the main carbon precursors used. In addition, the performance and characteristics of the resulting carbons are investigated. Finally, the contribution of activated carbon in the field of wastewater treatment will also be discussed in detail.
2. Classification of Porous Materials
According to the International Union of Pure and Applied Chemistry-USA(IUPAC) [33][34], porous materials are classified according to the diameter of the pores (dp) that constitute them, and the three classes are seen in Figure 1.
Figure 1. Classification of porosity based on pore diameter according to the International Union of Pure and Applied Chemistry (IUPAC).
2.1. Microporous Materials
Microporous materials are those whose pore diameter is less than 2 nm (dp<2 nm). They can be prepared by the sol-gel process and are widely used in catalysis and adsorption.
2.2. Mesoporous Materials
In this category of materials, the pore diameter varies between 2 and 50 nm (2 nm <dp<50 nm). A distinction is made between crystalline mesoporous materials and ordered amorphous materials, which are intermediate between that of crystalline microporous solids of the zeolite type and disordered amorphous solids such as silica gel [35][36][37]. Mesoporous materials have several known applications such as catalysis, filtration, pollution control, optics, and even electronics.
2.3. Macroporous Materials
As these materials have fairly large pores (dp> 50 nm), they find their use in the field of decontamination of polluted water with organic dyes and as a catalyst support for the photodegradation of the pollutant [38][39][40][41].
3. Graphite and Graphene
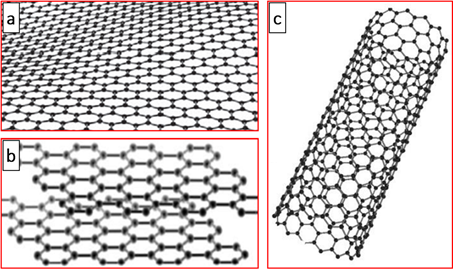
Graphite consists of interconnected carbon atoms arranged to hexagonally form flat networks in the form of layers stacked parallel to each other (Figure 2b)[42][43]. These layers are connected by low energy van der Waals type bonds. This explains the laminated morphology of graphite [42][43]. Graphite is characterized by high thermal and electrical conductivity, good chemical stability, and remarkable plasticity [43][44][45][46][47][48][49][50]. A good thermal and electrical conductivity is attributed to the delocalization of the π-electrons of the carbon atoms in graphite, and its plasticity can be explained by the possibility of sliding the sheets layers one onto the other [42][43]. These favorable characteristics make graphite interesting materials for improving the transfer and storage of thermal energy, for preparing anodes of lithium-ion batteries, and for electrochemical applications. In this context, Zhong et al. [51] used pitch-based graphite foams to increase the thermal diffusivity of paraffin wax for a thermal energy storage application. Later, Jana et al. [45] studied the effect of the addition of graphite on the conductivity of a tannin-based resin. They showed, contrary to what was expected a priori, that the smaller graphite particles were much more suitable for obtaining conductive matrices. Indeed, the use of small grains made the viscosity higher because of their higher surface and made it possible to obtain homogeneous foams with a higher density [45]. In 2019, Zhang et al. [52] showed that the use of graphitized mesoporous carbon as anode materials for lithium-ion batteries improved speed performance and had a good cycling stability with a reversible capacity of 248.3 mA h g−1 at 1 C after 100 cycles. In the same context, porous graphitic carbon was synthesized from Eichhornia crassipes plants collected from the Coimbatore region, Tamilnadu-India, and has been used for the sustainable fabrication of hole-transporting materials electrode in perovskite solar cells [53].
Figure 2. A conceptual model depicting the structure of graphene (a), graphite (b), and carbon nanotubes (c).
4. Carbon Nanotubes
Carbon nanotubes are an allotropic form of carbon (Figure 2c), observed for the first time in 1991 by Japanese researcher Sumio Iijima during the synthesis of fullerenes by an electric arc [54]. They have been characterized as graphene sheets that are wound on themselves in the form of microtubes and will later be renamed multiwall carbon nanotubes (MWCNTs). In 1993, the synthesis of single-walled carbon nanotubes (SWCNTs) was reported by optimizing the method of synthesis by an electric arc [55][56]. The multisheet nanotubes consist of several graphene sheets wound concentrically with a spacing between two sheets of approximately 3.6 Å, slightly greater than the spacing between two sheets in graphite. The diameter of the nanotubes varies, depending on the number of sheets, from 1 to 50 nm, and their lengths can reach 1 µm [57][58][59]. SWCNTs consist of a graphene sheet wound on itself, and they form a tube whose diameter is between 0.4 and 3 nm. Carbon nanotubes are therefore composed of one or more sheets of carbon atoms, as in graphite, wound on themselves forming a tube. Carbon nanotubes have good mechanical properties, good adhesion, and excellent electrical conductivity [43,111–113]. Some of these nanotubes have metallic properties [75,114,115], while others are semiconductors [60][61][62][63][64][65]. SWCNTs and MWCNTs are manufactured by almost the same method; the only distinction can appear on the use of the metal catalyst, generally nickel, iron, or cobalt, which is essential for the synthesis of fullerenes. They can be prepared mainly by arc discharge, laser ablation, and chemical vapor deposition [66][67][68][69][70], involving one of the following as carbon precursors: xylene, acetylene, toluene, methane, benzene,etc. [43]. On the industrial level, carbon nanotubes, with the particularity that they possess, residing in a small amount of impurities, are used in many forms such as the reinforcing elements of polymers and composites, nanoporous materials for energy storage, passive (nanometric conductors) or active components (diodes and transistors), and systems allowing for the vectorization of drug molecules for the treatment of certain diseases [64][64][65][66][71][72][73][74][75][76].
5. Activated Carbon
Activated carbon is porous, amorphous organic material with a complex structure, characterized by high carbon content [77]. These materials include a wide range of carbonaceous substances with different properties and characteristics (porosity, specific surface, chemical nature of the surface, density, etc.). Activated carbon can be produced from any substance with a high carbon content, whether of a vegetable, fossil, or material of a synthetic nature; examples include date stones [78][79], coffee grounds [80][81], almond shell [82], coconut shell [83], corncob wastes [84], Acacia glauca sawdust [85], waste potato residue [86][87], rice husk [88], sunflower piths [89], tomato stem [90], banana peel [91], etc.
This entry is adapted from the peer-reviewed paper 10.3390/pr8121651
References
- Rouquerol, F.; Luciani, L.; Llewellyn, P.; Denoyel, R.; Rouquerol, J. Techniques de L’ingénieur, Analyse et Caractérisation: Texture des Matériaux Pulvérulents ou Poreux; Inist-CNRS-Paris: Paris, France, 2003; pp. 1050–1057. [Google Scholar]
- Besson, J.; Billon, N.; Cantournet, S.; Chastel, Y.; Lorenzon, A.F.G.; Haudin, J.M.; Monasse, B.; Naze, L. Matériaux pour l’ingénieur; Edition Mines: Paris, France, 2006; pp. 81–82. [Google Scholar]
- Ma, T.Y.; Yuan, Z.Y. Bioinspired Approach to Synthesizing Hierarchical Porous Materials, Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science, 1st ed.; Su, B.L., Sanchez, C., Yang, X.Y., Eds.; Wiley: Weinheim, Germany, 2012; pp. 173–207. [Google Scholar]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high performance supercapacitor and CO2 capture. Ind. Crop. Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Menéndez, J.A.; Arenillas, A. Performance of carbon xerogel-graphene hybrids as electrodes in aqueous supercapacitors. Electrochim. Acta 2018, 276, 28–36. [Google Scholar]
- Sarvi, M.N.; Bee, T.B.; Gooi, C.K.; Woonton, B.W.; Gee, M.L.; O’Connor, A.J. Development of functionalized mesoporous silica for adsorption and separation of dairy proteins. Chem. Eng. J. 2014, 235, 244–251. [Google Scholar] [CrossRef]
- Xin, Y.; Peng, S.; Chen, J.; Yang, Z.; Zhang, J. Continuous flow synthesis of porous materials. Chin. Chem. Lett. 2019, 31, 1448–1461. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zecevic, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-Free Oxidation of Alcohols to Esters at Room Temperature and Atmospheric Conditions using Nanoscale Co-Based Catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Xu, L.; Guo, L.; Hu, G.; Chen, J.; Hu, X.; Wang, S.; Dai, W.; Fan, M. Nitrogen-doped porous carbon spheres derived from d-glucose as highly-efficient CO2 sorbents. RSC Adv. 2015, 5, 37964–37969. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zheng, M.; Zhao, H.; Zhao, Y.; Sun, Z. Hierarchically structured porous nitrogen-doped carbon for highly selective CO2 capture. ACS Sustain. Chem. Eng. 2016, 4, 298–304. [Google Scholar] [CrossRef]
- Xu, A.-R.; Chen, L.; Guo, X.; Xiao, Z.; Liu, R. Biodegradable lignocellulosic porous materials: Fabrication, characterization and its application in water processing. Int. J. Biol. Macromol. 2018, 115, 846–852. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.; Zhang, W.; Zhang, Y. One-pot synthesis of porous carbon foam derived from corn straw: Atrazine adsorption equilibrium and kinetics. Environ. Sci. Nano. 2017, 4, 625–635. [Google Scholar] [CrossRef]
- Little, I.; Alorkpa, E.; Khan, V.; Povazhnyi, V.; Vasiliev, A. Efficient porous adsorbent for removal of cesium from contaminated water. J. Porous Mater. 2019, 26, 361–369. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, H.; Li, W.; Zhang, X. Resorcinol-formaldehyde based porous carbon as an electrode material for supercapacitors. Carbon 2007, 45, 160–165. [Google Scholar] [CrossRef]
- Zhang, W.F.; Huang, Z.-H.; Cao, G.P.; Kang, F.; Yang, Y. A novel mesoporous carbon with straight tunnel-like pore structure for high rate electrochemical capacitors. J. Power Sources 2012, 204, 230–235. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Q. Nitrogen-doped mesoporous carbons for high performance supercapacitors. Appl. Surf. Sci. 2016, 379, 132–139. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Xu, F.; Zhao, W.; Sun, P.; Zhang, Z.; Qian, M.; Huang, F. Constructing hierarchical porous carbon via tin punching for efficient electrochemical energy storage. Carbon 2018, 134, 391–397. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Hu, H.; Liu, S.; Cai, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Ultrahigh-surface-area hierarchical porous carbon from chitosan: Acetic acid mediated efficient synthesis and its application in superior supercapacitors. J. Mater. Chem. 2017, 5, 24775–24781. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Evans, S.D.; Ge, Y.; Zhu, Z.; Tu, Y.; Yang, X. Confined assembly of hollow carbon spheres in carbonaceous nanotube: A spheres-in-tube carbon nanostructure with hierarchical porosity for high-performance supercapacitor. Small 2018, 14, 1704015. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Baik, S.; Lee, J.W. Spent coffee derived hierarchical porous carbon and its application for energy storage. J. Porous Mater. 2020, 27, 451–463. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Yang, Y.; Wang, H.; Yan, F.-Y.; Qi, Y.; Lai, Y.-K.; Zeng, D.-M.; Chen, G.; Zhang, K.-Q. Bioinspired Porous Octacalcium Phosphate/Silk Fibroin Composite Coating Materials Prepared by Electrochemical Deposition. ACS Appl. Mater. Interfaces 2015, 7, 5634–5642. [Google Scholar] [CrossRef] [PubMed]
- Pallares, J.; Cencerrado, A.G.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Siddique, A.; Nayak, A.K.; Singh, J. Synthesis of FeCl3-activated carbon derived from waste Citrus limetta peels for removal of fluoride: An eco-friendly approach for the treatment of groundwater and bio-waste collectively. Groundw. Sustain. Dev. 2020, 10, 100339. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Chen, J.; Lin, J.-H. Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J. Environ. Chem. Eng. 2020, 8, 103929. [Google Scholar] [CrossRef]
- Giraldo, S.; Robles, I.; Ramirez, A.; Florez, E.; Acelas, N. Mercury removal from wastewater using agroindustrial waste adsorbents. SN Appl. Sci. 2020, 2, 1029. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mnasri, N.; Charnay, C.; Ménorval, L.C.; Moussaoui, Y.; Elaloui, E.; Zajac, J. Silver nanoparticle-containing submicron-in-size mesoporous silica-based systems for iodine entrapment and immobilization from gas phase. Microporous Mesoporous Mater. 2014, 196, 305–313. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Jesus, R.A.; Santos, D.; Manoa, J.F.; Romao, L.P.C.; Paranhos, C.M. Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microporous Mesoporous Mater. 2020, 291, 109698. [Google Scholar] [CrossRef]
- Luo, X.; Guoa, J.; Chang, P.; Qian, H.; Pei, F.; Wang, W.; Miao, K.; Guo, S.; Feng, G. ZSM-5@MCM-41 composite porous materials with a core-shell structure: Adjustment of mesoporous orientation basing on interfacial electrostatic interactions and their application in selective aromatics transport. Sep. Purif. Technol. 2020, 239, 116516. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, P.; Liu, S.; Yu, K. Three-dimensionally ordered macroporous perovskite materials for environmental applications. Chin. J. Catal. 2019, 40, 1324–1338. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Pan, Y.; Xu, M.; Yang, L.; Qu, Y.; Guo, M.; Yuan, C. Graphene-templated growth of vertical MnO nanosheets with open macroporous architectures as anode materials for fast lithium storage. J. Alloys Compd. 2018, 769, 10–17. [Google Scholar] [CrossRef]
- Simaioforidou, A.; Kostas, V.; Karakassides, M.A.; Louloudi, M. Surface chemical modification of macroporous and mesoporous carbon materials: Effect on their textural and catalytic properties. Microporous Mesoporous Mater. 2019, 279, 334–344. [Google Scholar] [CrossRef]
- Lal, S.; Prat, M.; Plamondon, M.; Poulikakos, L.; Partl, M.N.; Derome, D.; Carmeliet, J. A cluster-based pore network model of drying with corner liquid films, with application to a macroporous material. Int. J. Heat Mass Transf. 2019, 140, 620–633. [Google Scholar] [CrossRef]
- Deprez, N.; McLachlan, D.S. The analysis of the electrical conductivity of graphite conductivity of graphite powders during compaction. J. Phys. D Appl. Phys. 1988, 21, 101–107. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Fan, L.; Li, S.; Sari, H.M.K.; Qin, J. A Review of Carbon-Based Materials for Safe Lithium Metal Anodes. Front. Chem. 2019, 7, 721. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Pizzi, A.; Celzard, A. Thermal conductivity improvement of composite carbon foams based on tannin-based disordered carbon matrix and graphite fillers. Mater. Des. 2015, 83, 635–643. [Google Scholar] [CrossRef]
- Dong, Y.; Meng, M.; Groves, M.M.; Zhang, C.; Lin, J. Thermal conductivities of two-dimensional graphitic carbon nitrides by molecule dynamics simulation. Int. J. Heat Mass Transf. 2018, 123, 738–746. [Google Scholar] [CrossRef]
- Duan, J.; Wu, W.; Nolan, A.M.; Wang, T.; Wen, J.; Hu, C.; Mo, Y.; Luo, W.; Huang, Y. Lithium graphite paste: An interface compatible anode for solid-state batteries. Adv. Mater. 2019, 31, 1807243. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-M.; Chen, H.; Chen, Y.-Z.; Li, P.; Zhang, J.-Q.; Zhao, X. Simple synthesis of porous carbon materials for high-performance supercapacitors. J. Appl. Electrochem. 2016, 46, 703–712. [Google Scholar] [CrossRef]
- Li, G.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Yang, Z.; Liu, Y.; Ji, X.; Wang, J. Metalorganic Quantum Dots and Their Graphene-Like Derivative Porous Graphitic Carbon for Advanced Lithium-Ion Hybrid Supercapacitor. Adv. Energy Mater. 2019, 9, 1802878. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Liu, Y.; Li, L.; Wang, J. Non-aqueous dual-carbon lithium-ion capacitors: A review. J. Mater. Chem. A 2019, 7, 15541–15563. [Google Scholar] [CrossRef]
- Zhong, Y.; Guo, Q.; Li, S.; Shi, J.; Liu, L. Heat transfer enhancement of paraffin wax using graphite foam for thermal energy storage. Sol. Energy Mater. Sol. Cells 2010, 94, 1011–1014. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.; Cong, Y.; Zhang, Y.; Li, X.; Dong, Z.; Li, Y.; Yuan, G.; Zhang, J.; Cui, Z. Improved rate performance and cycling stability of graphitized mesoporous carbon as anode materials for lithium-ion batteries. J. Mater. Sci. 2019, 54, 648–658. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Eswaramoorthy, N.; Natarajan, M.; Santhanam, A.; Asokan, V.; Ramakrishnan, V.M.; Rangasamy, B.; Sundaram, S.; Ravirajan, P.; Velauthapillai, D. Perovskite Solar Cells: A Porous Graphitic Carbon based Hole Transporter/Counter Electrode Material Extracted from an Invasive Plant Species Eichhornia Crassipes. Sci. Rep. 2020, 10, 6835. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bethune, D.S.; Klang, C.H.; De Vries, M.S.; Gorman, G.; Savoy, R.; Vasquez, J.; Beyers, R. Cobalt catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Inagaki, M. Advanced Carbon Materials. In Handbook of Advanced Ceramics, 2nd ed.; Somiya, S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 25–60. [Google Scholar]
- Gupta, S.; Tai, N.H. Carbon materials and their composites for electromagnetic interference shielding effectiveness in X-band. Carbon 2019, 152, 159–187. [Google Scholar] [CrossRef]
- Lee, D.W.; Park, J.; Kim, B.J.; Kim, H.; Choi, C.; Baughman, R.H.; Kim, S.J.; Kim, Y.T. Enhancement of electromagnetic interference shielding effectiveness with alignment of spinnable multiwalled carbon nanotubes. Carbon 2019, 142, 528–534. [Google Scholar] [CrossRef]
- Rutherglen, C.; Burke, P. Nanoelectromagnetics: Circuit and Electromagnetic Properties of Carbon Nanotubes. Small 2009, 5, 884–906. [Google Scholar] [CrossRef]
- Daneshvar, F.; Aziz, A.; Abdelkader, A.M.; Zhang, T.; Sue, H.-J.; Welland, M.E. Porous SnO2–CuxO nanocomposite thin film on carbon nanotubes as electrodes for high performance supercapacitors. Nanotechnology 2018, 30, 015401. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, G.; Ibanez, J.; Rojo, J.M.; Kunowsky, M. Activated carbon fiber monoliths as supercapacitor electrodes. Adv. Mater. Sci. Eng. 2017, 1–8. [Google Scholar] [CrossRef]
- Ma, W.L.; Cai, Z.H.; Zhang, Y.; Wang, Z.Y.; Xia, L.; Ma, S.P.; Lia, G.H.; Huang, Y. An Overview of Stretchable Supercapacitors Based on Carbon Nanotube and Graphene. Chin. J. Polym. Sci. 2020, 38, 491–505. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Duong, H.; Cheng, H.; Jewell, D. Advanced Supercapacitors Using Carbon Nanotubes. Mater. Res. Found. 2017, 12, 207–228. [Google Scholar]
- Ying, L.S.; Mohd Salleh, M.A.; Yusoff, H.M.; Rashid, S.B.A.; Razak, J. Continuous production of carbon nanotubes. J. Ind. Eng. Chem. 2011, 17, 367–376. [Google Scholar] [CrossRef]
- Tai, N.H.; Chen, H.M.; Chen, Y.J.; Hsieh, P.Y.; Liang, J.R.; Chou, T.W. Optimization of processing parameters of the chemical vapor deposition process for synthesizing high-quality single-walled carbon nanotube fluff and roving. Compos. Sci. Technol. 2012, 72, 1855–1862. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Selfassembly of tubular fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Zhou, D.; Anoshkina, E.V.; Chow, L.; Chai, G. Synthesis of carbon nanotubes by electrochemical deposition at room temperature. Carbon 2006, 44, 1013–1016. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, Y.; Ubnoske, S.; Zang, J.; Cao, Y.; Henry, P.; Parker, C.B.; Glass, J.T. Highly stretchable supercapacitors via crumpled vertically aligned carbon nanotube forests. Adv. Energy Mater. 2019, 9, 1900618. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Li, Y.; Wang, Y.; Zhang, J.; Guan, G.; Pan, Z.; Zheng, G.; Peng, H. Nitrogen-doped core-sheath carbon nanotube array for highly stretchable supercapacitor. Adv. Energy Mater. 2017, 7, 1601814. [Google Scholar] [CrossRef]
- Hua, C.; Shang, Y.; Wang, Y.; Xu, J.; Zhang, Y.; Li, X.; Cao, A. A flexible gas sensor based on single-walled carbon nanotube-Fe2O3 composite film. Appl. Surf. Sci. 2017, 405, 405–411. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors, Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Ratna Prabha, C. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef]
- Farghali, A.A.; Tawab, H.A.A.; Moaty, S.A.A.; Khaled, R. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J. Nanostruct. Chem. 2017, 7, 101–111. [Google Scholar] [CrossRef]
- Getachew, T.; Hussen, A.; Rao, V.M. Defluoridation of water by activated carbon prepared from banana (Musa paradisiaca) peel and coffee (Coffe arabica) husk. Int. J. Environ. Sci. Technol. 2015, 12, 1857–1866. [Google Scholar] [CrossRef]
- Darweesh, T.M.; Ahmed, M.J. Batch and fixed bed adsorption of levofloxacin on granular activated carbon from date (Phoenix dactylifera L.) stones by KOH chemical activation. Environ. Toxicol. Pharmacol. 2017, 50, 159–166. [Google Scholar] [CrossRef]
- Khadhri, N.; Saad, M.E.K.; ben Mosbah, M.; Moussaoui, Y. Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Laksaci, H.; Khelifi, A.; Belhamdi, B.; Trari, M. Valorization of coffee grounds into activated carbon using physic—Chemical activation by KOH/CO2. J. Environ. Chem. Eng. 2017, 5, 5061–5066. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ngo, H.H.; Guo, W.S. Preparation of a specific bamboo based activated carbon and its application for ciprofloxacin removal. Sci. Total Environ. 2015, 533, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.; Salari, K.; Yazdi, Z.A. Preparation of activated carbon from agricultural wastes (almond shell and orange peel) for adsorption of 2-pic from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 1892–1900. [Google Scholar] [CrossRef]
- Talat, M.; Mohan, S.; Dixit, V.; Singh, D.K.; Hasan, S.H.; Srivastava, O.N. Effective removal of fluoride from water by coconut husk activated carbon in fixed bed column: Experimental and breakthrough curves analysis. Groundwater Sustainable Dev. 2018, 7, 48–55. [Google Scholar] [CrossRef]
- Vu, M.T.; Chao, H.-P.; Trinh, T.V.; Le, T.T.; Lin, C.-C.; Tran, H.N. Removal of ammonium from groundwater using NaOH-treated activated carbon derived from corncob wastes: Batch and column experiments. J. Clean. Prod. 2018, 180, 560–570. [Google Scholar] [CrossRef]
- Dhorabe, P.T.; Lataye, D.H.; Ingole, R.S. Removal of 4-nitrophenol from aqueous solution by adsorption onto activated carbon prepared from Acacia glauca sawdust. Water Sci. Technol. 2016, 73, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, X.; Liu, Y.; Zhou, P.; Ma, G.; Lei, Z.; Lei, L. A low cost and highly efficient adsorbent (activated carbon) prepared from waste potato residue. J. Taiwan Inst. Chem. Eng. 2015, 49, 206–211. [Google Scholar] [CrossRef]
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 37228–37241. [Google Scholar] [CrossRef]
- Satayeva, A.R.; Howell, C.A.; Korobeinyk, A.V.; Jandosov, J.; Inglezakis, V.J.; Mansurov, Z.A.; Mikhalovsky, S.V. Investigation of rice husk derived activated carbon for removal of nitrate contamination from water. Sci. Total Environ. 2018, 630, 1237–1245. [Google Scholar] [CrossRef]
- Baysal, M.; Bilge, K.; Yilmaz, B.; Papila, M.; Yürüm, Y. Preparation of high surface area activated carbon from waste-biomass of sunflower piths: Kinetics and equilibrium studies on the dye removal. J. Environ. Chem. Eng. 2018, 6, 1702–1713. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Wang, Y.; Li, Q. Activated carbon from tomato stem by chemical activation with FeCl2. Colloids Surf. A 2017, 529, 842–849. [Google Scholar] [CrossRef]
- Liew, R.K.; Azwar, E.; Yek, P.N.Y.; Lim, X.Y.; Cheng, C.K.; Ng, J.-H.; Jusoh, A.; Lam, W.H.; Ibrahim, M.D.; Ma, N.L.; et al. Microwave pyrolysis with KOH/NaOH mixture activation: A new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Bioresour. Technol. 2018, 266, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baysal, M.; Bilge, K.; Yilmaz, B.; Papila, M.; Yürüm, Y. Preparation of high surface area activated carbon from waste-biomass of sunflower piths: Kinetics and equilibrium studies on the dye removal. J. Environ. Chem. Eng. 2018, 6, 1702–1713. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Wang, Y.; Li, Q. Activated carbon from tomato stem by chemical activation with FeCl2. Colloids Surf. A 2017, 529, 842–849. [Google Scholar] [CrossRef]
- Liew, R.K.; Azwar, E.; Yek, P.N.Y.; Lim, X.Y.; Cheng, C.K.; Ng, J.-H.; Jusoh, A.; Lam, W.H.; Ibrahim, M.D.; Ma, N.L.; et al. Microwave pyrolysis with KOH/NaOH mixture activation: A new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Bioresour. Technol. 2018, 266, 1–10. [Google Scholar] [CrossRef] [PubMed]