1. The Pathophysiology of Inflammatory Colon Disease
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a global health concern because of the burdens of medical costs attributable to their acute and chronic complications and the poor quality of life of patients [
1,
2]. IBD has a complex etiology and pathophysiologic factors, including genetic variants, an inappropriate inflammatory response to the gut microbiome, dysregulation of immune-modulated intestinal inflammation, and environmental factors [
3,
4,
5]. Although the pathogenesis of IBD is complicated and the clinical presentations of UC and CD are distinct, there are common phenotypes, such as the deregulation of colonic immune response and chronic inflammation, implicated in the pathogenesis of both subtypes [
6,
7,
8]. Therefore, much of the research on IBD pathogenesis and therapeutic investigation has focused on the immune system and its maintenance of homeostasis processes.
During perturbation of the integrity of the intestinal epithelial barrier and the gut lumen defects, microbial translocation occurs, which promotes activation of the innate immune cells such as macrophages and dendritic cells in the lamina propria. Antigen-presenting cells then activate naïve T cells and promote their development into different types of effector CD4
+ cells, which alter gut homeostasis. The development of pathogenic T cells is linked with the environmental factors correlated with IBD pathology, such as tissue metabolism and the production of pro-inflammatory cytokines. Moreover, the development of chronic colon inflammation occurs when uncontrolled infiltration of inflammatory CD4
+ T cells into the lamina propria of patients with IBD because of their dysfunctional barrier and defeat in immune tolerance for intestinal antigens [
8,
9]. Current studies reveal that T helper (Th) cells are major modulators of intestinal colitogenesis [
10]. Th cells responding to T-cell receptor-mediated activation by a range of pathogens and the stimulation of cytokines can differentiate into several Th cell lineages, which result in distinct effector subsets, including T Th1, Th2, Th17, and regulatory T cells (Treg), which play important roles in the immune response [
11]. Genome-wide association studies have demonstrated that single-nucleotide polymorphisms in genes encoding transcription factors modulate cytokine-mediated regulation of the immune responses correlated with the pathogenesis of IBD [
4,
12]. The immunological pathogenesis of IBD involves an altered immune response with the pronounced infiltration of adaptive immune cells into the lamina propria of the intestines, and the augmented expression of mucosal pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and the IL-12/IL-23 pathways [
9,
13,
14,
15,
16].
2. The Effect of Obesity on the Development of IBD
The incidence of IBD is augmenting in North America, Europe, and Asia, and is speeding in newly industrialized countries [
2,
17]; the prevalence of overweight and obesity is also growing in parallel to the IBD pandemic [
18]. The incidence of IBD and obesity have increased markedly in countries in which the lifestyle changes have included diets rich in animal fat and low in dietary fiber and insufficient physical activity [
19,
20,
21,
22]. Obesity significantly involves an expansion of the entire volume of fat tissue accompanied by distinct alterations in the balance of the cellular and humoral immunity [
23]. The expression of pro-inflammatory cytokines and the infiltration of adipose tissue by immune cells such as Tregs and macrophages are augmented in obese compared with lean individuals [
23]. Moreover, obesity is correlated with a chronic inflammatory state, illustrated by the signaling pathway activation of inflammation, enhanced synthesis of C-reactive protein and pro-inflammatory cytokines, and activation of the pro-inflammatory transcription factors in adipocytes compared with that in healthy controls [
24,
25]. These changes in adipose tissue and their metabolic and systemic consequences have contributed to the model of obesity as an inflammatory state [
26]. This chronic inflammation eventually leads to intestinal inflammatory diseases. Visceral adiposity is particularly correlated with the development of insulin resistance and associates with metabolic syndrome [
27]. Observational studies have reported that around 20%–30% of children with IBD are overweight, while prior IBD-related surgery was correlated with obesity in these pediatric patients with CD [
28]. The same phenomenon was observed in adult patients with IBD in Tayside Scotland: only a small number of patients with IBD were underweight, whereas 18% of the population with CD were obese and 52% were overweight, demonstrating that the incidence of IBD significantly increased in obese patients [
29]. Furthermore, previous studies reported that the development of obesity is critically associated with alterations of in the gut microbiota, which, in turn, impact mucosal immunity and intestinal inflammation [
30,
31].
The pro-inflammatory state of visceral obesity is associated with several gastrointestinal diseases, such as IBD and fatty liver disease, and a crucial correlation noted between IBD and obesity concerns non-alcoholic fatty liver disease (NAFLD) [
32]. It has been reported that the prevalence of NAFLD is augmented among IBD patients as compared to non-IBD controls, and this phenomenon occurs commonly at a young age [
33]. Moreover, the diagnosis of NAFLD by transient elastography with controlled attenuation parameters is a common comorbidity for IBD patients and is correlated with extrahepatic diseases [
34]. These results suggest that the perpetuation of inflammation promotes risks for co-morbid conditions shared between IBD and NAFLD, and these developments could link the epidemiology of these diseases.
3. The Interaction of Adipose Tissue and the Intestinal Immune System
Adipose tissue, which was initially considered to be merely an energy store, is now recognized as an endocrine organ that not only stores energy but also secretes or produces various biologically active substances called adipokines [
35] and interacts closely with the immune system [
36,
37]. Thus, adipose tissue is now regarded as an endocrine organ with several functions [
38]. Adipose tissue can be separated into subcutaneous and visceral adipose tissue (VAT), the relative amounts of which vary greatly between individuals, and the total body weight ranges from 5% to 60% [
39]. Adipose tissue is composed of multiple cell types, of which adipocytes are the most prominent, followed by vascular endothelial cells, macrophages [
40,
41], and lymphocytes [
42,
43], which are found in the stromovascular fraction. It has been reported that pediatric patients with CD were observed with higher VAT volumes than healthy controls and the odds of CD-related hospitalization was correlated with the increase in VAT volume [
44], suggesting that obesity is a critical risk factor related to the development of CD in pediatric patients; VAT could be a better predictor of disease progression than obesity determined by body mass index (BMI) and is a possible marker of the progress of pro-inflammation or metabolic activity [
45,
46]. Studies in patients with CD by performing visceral adiposity as the obesity quantity have more consistently shown an augmented risk of complications with CD than those using BMI as the obesity marker [
47,
48,
49]. Computed tomography for mesenteric fat index (MFI) have suggested that the ratio of the area of VAT to that of subcutaneous fat is a marker for the CD progression: VAT area and MFI values were correlated with the postoperative recurrence of CD and the ratio of VAT to subcutaneous adipose tissue was associated more strongly than BMI with the increase in disease activity of CD and its structural behavior [
50].
Adipocytes comprise white and brown cells, presumably including diverse intermediate forms. Because white fat cells are the critical part in adipose tissues from adults [
51,
52], this review focuses on these. Mature white adipocytes consist of a large fat droplet surrounded by a margin of remaining cytoplasm and the nucleus. Adipocytes are characterized by their cellular plasticity, store the body’s energy supplies, and actively secrete various adipokines [
53]. Macrophages and T cells are the two most common immune cells present in adipose tissue. Altered infiltration of immune cells and the dysregulated production of pro-inflammatory cytokines have been documented in obesity [
41,
54,
55,
56]. For example, adipose tissue-resident T cells increase approximately threefold in the high-fat diet (HFD) mouse model of obesity [
57]. The adipokines comprise a group of mediators secreted by adipose tissue, including adiponectin, leptin, apelin, and resistin. Recent evidence indicates that adipokines can modulate immune functions in addition to their regulation of metabolic homeostasis within adipose tissue [
25,
58]. For example, previous studies have demonstrated that adiponectin can suppress the phagocytic activity of mature macrophages mediated by the complement C1q receptor and inhibit TNF-α production stimulated by lipopolysaccharide (LPS) [
36]. In addition, leptin has been reported to be a T-cell immune response modulator that can enhance the cytotoxicity of natural killer (NK) cells, induce activation of granulocytes, macrophages and dendritic cells, and polarize Th cell subsets differentiation toward a pro-inflammatory Th1 phenotype rather than an anti-inflammatory Th2 phenotype [
37,
59]. Thus, investigation of the adipokine modulation of immune responses as a potential therapeutic target to modulate inflammation in the context of the pathophysiology of IBD may be a focus of future research in inflammatory colon diseases.
Evidence has demonstrated that excessive adipose tissue accumulation, which is characterized by a chronic inflammatory state involving the activation of pro-inflammatory signaling pathways and increased pro-inflammatory cytokine production [
24], increases the risks of developing many chronic diseases, including UC and CD [
24,
60,
61,
62,
63]. Recent reports have demonstrated a particular role of mesenteric adipose tissue that migrates around intestine, so-called “creeping fat”. The presence of creeping fat, in which the expression of peroxisome proliferator-activated receptor (PPAR)-γ and TNF-α is augmented [
64], possesses an association with transmural inflammation, fibrosis, and stricture formation [
65], which lead to pathogenic intestinal inflammation in patients with CD [
45,
66]. Moreover, recent epidemiological analyses have demonstrated the influence of the Western diet on changes in body composition and aggravation of colitis severity in patients with IBD [
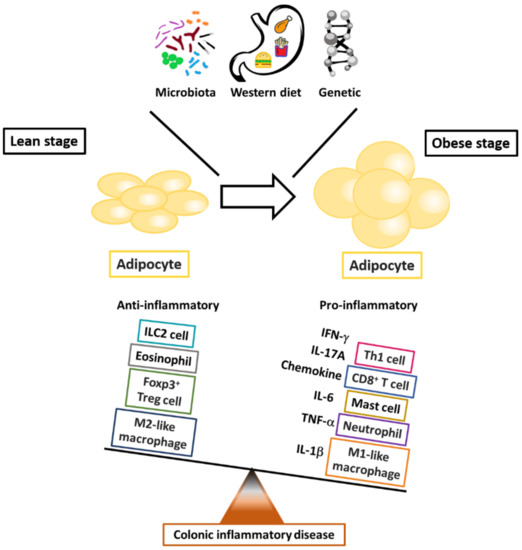
20]. Thus, both in vitro and in vivo studies and epidemiologic research indicate the potential role in inflammatory colon diseases of adipokine-modulated immune regulation of the interplay between adipose tissue, chronic inflammation, and immune cells (). This review focuses on studies that relate immune cells, adipose tissue, and the selected adipokine-modulated production of pro-inflammatory cytokines to immunologic homeostasis in inflammatory colon diseases.
Figure 1. The critical roles of immune cells in fat-associated colonic inflammation. The outcome of obesity-modulated inflammation in adipose tissue reflects the balance between pro- and anti-inflammatory mediators in the systems of innate and adaptive immune cells.