Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders of the gastrointestinal tract whose etiology is unknown. Pathogenesis of IBD is attributed to the complex interaction of genetic susceptibility, environmental factors (such as smoking, diet, and infections), and the gut microbiota. This results in an uncontrolled immune response leading to mucosal damage. IBDs are characterized by a relapsing and remitting course and require lifelong treatment. Therapy aims to induce remission, maintain remission, and avoid disease progression.
Fecal markers are a heterogeneous group of biological substances formed by the inflamed intestinal mucosa or pass through it and enter the intestinal lumen and feces, where they can be measured. The advantage of fecal markers of inflammation over blood markers is that they provide information about the inflammatory process’s location, particularly the location along the gastrointestinal tract. Still, they also are not specific to IBD.
1. Fecal Alpha-1 Antitrypsin
Alpha-1 antitrypsin (AAT) is a serum protease inhibitor that constrains and modifies the effect of 90% of serum proteolytic enzymes—trypsin, neutrophil elastase, pancreatic elastase, serine protease, collagenase, kallikrein, and factor-8. Unlike other major serum proteins, it is highly resistant to intestinal protein degradation and is excreted intact in the feces [
44]. In the presence of intestinal inflammation, due to increased permeability and impaired mucosal integrity, AAT excretion in the feces increases [
45]. Elevated fecal AAT levels are found in protein-losing enteropathies, celiac disease, giardiasis, IBD, malignancies, etc. Various studies have been performed in patients with IBD to assess the relationship between fecal AAT concentration and disease activity. However, the results are contradictory [
46,
47,
48,
49].
2. Fecal Lactoferrin
Lactoferrin is an 80 kDa iron-binding protein. It is secreted by specific epithelial cells and can be found in various bodily fluids, including serum, tears, synovial fluid, and breast milk [
50,
51]. Besides, lactoferrin is the major protein in the secondary granules of polymorphonuclear granulocytes. It has both proinflammatory and anti-inflammatory properties [
52]. In the presence of intestinal inflammation, polymorphonuclear cells infiltrate into the intestinal mucosa and subsequently enter the intestinal lumen, which results in a rise of the concentration of lactoferrin in the feces [
53].
Fecal lactoferrin (FL) is stable for up to 5 days at room temperature. Its presence can be determined by qualitative and quantitative tests [
54,
55]. FL testing is used both in the diagnosis and in the follow-up of patients with IBD [
54,
56]. Its limitations as a marker in IBD are related to its non-specificity—elevated levels of FL are also found in various intestinal infections, during the administration of non-steroidal anti-inflammatory drugs (NSAIDs), in malignant intestinal diseases, etc.; to the fact that, in addition to neutrophils, it is secreted by other cells; and to the evidence of its lower accuracy as a laboratory indicator compared to fecal calprotectin (FC), a fecal marker that is most widely studied and used in clinical practice [
57,
58,
59].
3. Fecal Calprotectin
Calprotectin is a small calcium and zinc-binding protein with a molecular weight of 36 kDa [
60]. It was discovered in 1980 by Magne Fagerhol et al. and was initially named the L1 protein or leukocyte-derived L1 protein [
61]. The name calprotectin was proposed after it was found to have antimicrobial (protective) properties and given its ability to bind calcium [
62]. Calprotectin is a heterocomplex consisting of one light (8 kDa) and two heavy chains (14 kDa each), which are non-covalently linked [
63]. It belongs to the group of S100 proteins [
64]. S100 are low molecular weight proteins that bind calcium and participate in many intracellular and extracellular processes, including enzyme activation, cell growth, differentiation, inflammatory responses, etc. [
65]. The genes encoding calprotectin and other S100 proteins are located in the long arm of chromosome 1q12–q21 [
64].
Calprotectin is found predominantly in neutrophils, monocytes, and macrophages [
66,
67,
68]. It makes up 5% of the total amount of protein and approximately 60% of the cytosolic proteins in neutrophils. In monocytes, it is approximately 1.6% of the total amount of protein [
61,
63]. Calprotectin features antimicrobial and antifungal activity [
62,
69]. Additionally, it inhibits cell growth and induces cell death in specific cell types such as fibroblasts, some tumor cells, and others [
70,
71,
72]. Thus, as a result of its biological functions, it participates actively in regulating the inflammatory process. Upon stimulation, neutrophils and monocytes secrete calprotectin extracellularly [
66]. Its extracellular release is also observed in cell destruction or cell death [
73]. Typically, calprotectin can be found in plasma, synovial fluid, cerebrospinal fluid, saliva, urine, and feces [
74,
75]. An elevated level of calprotectin has been observed with increased accumulation of inflammatory cells in the course of an infection, some other inflammatory process, or malignancies [
74].
In intestinal inflammation, the concentration of calprotectin in the feces is proportional to the degree of neutrophil infiltration into the intestinal wall, and the number of neutrophils in the intestinal lumen [
76]. Elevated levels of FC are found in IBD, polyps, intestinal malignancies, intestinal infections, and others [
75,
76,
77]. The use of NSAIDs and proton pump inhibitors and bleeding outside the gastrointestinal tract also elevates FC levels. Its concentration is physiologically higher in early childhood [
77]. FC is homogeneously distributed in feces and is stable for up to 1 week at room temperature [
75]. Significant differences in its concentration may be observed if measured sequentially on different days [
78].
In recent years, FC has been widely studied as a marker in IBD. Its importance for diagnosing IBD, its role in monitoring patients with IBD to assess the disease activity and the response to therapy, and its ability to predict clinical recurrence or mucosal healing have been studied. The results generally show that FC helps to diagnose patients with IBD and facilitate patient follow-up by providing information on disease activity, the presence or absence of response to treatment, remission achieved, or recurrence risk [
79]. The specific sensitivity and specificity of this indicator and the different correlation indices vary widely between the studies and depend on the studied population and the cut-off values used [
43,
79,
80].
4. M2-Pyruvate Kinase
It was shown that fecal M2-PK concentrations are elevated in colorectal carcinoma and intestinal inflammation [
81], indicating increased cell turnover. Moreover, it is believed that intestinal epithelial cells may be resistant to apoptosis by upregulation of M2-PK via the Bcl-xl pathway in CD [
82].
Czub et al. tested fecal M2-PK levels in 107 Polish children with IBD (75 with UC, 32 with CD, and 35 healthy controls) [
83]. M2-PK levels in stool samples were higher in children with IBD and were associated with the pediatric Crohn’s disease activity index (PCDAI). While mean M2-PK levels were higher in those with active disease, 47% of children with IBD deemed to be in remission still had elevated M2-PK levels. Another study, conducted by Day et al. (2012), also demonstrated higher fecal M2-PK in CD children but without an established association with PCDAI scores or serum inflammatory markers, such as fecal S100A12 [
84]. Interestingly, children with ileocolonic disease appeared to have higher concentrations of M2-PK than those with isolated colonic or ileal disease.
Enhanced fecal M2-PK levels have also been seen in children with active UC [
85]. Furthermore, M2-PK was superior to other markers (calprotectin, S100A12, and lactoferrin). In a 2014 study by Czub et al., when using Truelove–Witts score for UC patients and PCDAI for CD patients, M2-PK concentration was identified as inferior to calprotectin, especially in children in IBD remission. However, on the contrary, are the findings made by Roszak et al. [
86] who suggest that M2-PK is a more sensitive marker to assess disease activity in pediatric UC or CD than calprotectin and lactoferrin. More studies are needed to explain this difference in this field.
5. Osteoprotegerin
Osteoprotegerin (OPG) belongs to the TNF superfamily and represents a cytokine receptor. A broad variety of cell types, distinct from those that produce calprotectin and lactoferrin, produce OPG—osteoblasts, B lymphocytes, dendritic cells, stromal bone marrow cells, epithelial cells, and monocytes/macrophages [
82]. It is thought that OPG promotes bone formation, contrary to inflammatory cytokines such as IL-1, TNFα, etc. This is especially important for pediatric IBD, where the increased risk of fracture associated with the disease is described. Nevertheless, the role of OPG produced in the intestinal mucosa on bone loss in the IBD remains unresolved. Besides its bone turnover role, OPG exerts functions in IBD pathogenesis related to local and systemic inflammation [
82].
A limited number of studies have shown that OPG is a useful biomarker of inflammation in pediatric IBD. Nahidi et al. [
87] measured OPG in children with CD. Serum, stool, and biopsy samples showed dramatically rise in the OPG levels. Moreover, the remission after induction therapy dropped the serum and fecal levels substantially. Those children with isolated colonic CD had higher levels than those with ileocolonic form. However, serum and fecal of OPG did not correlate with the PCDAI scores, but with CRP and fecal S100A12 before and not after treatment.
Regarding pediatric UC, Sylvester et al. [
88] assessed fecal OPG as a predictive marker for children’s treatment response. They demonstrated that patients with failed first-line corticosteroid therapy or who required infliximab or colectomy had elevated fecal OPG. Thus, OPG was superior to lactoferrin or S100A12 in predicting the treatment response. This gives us hope that it can be used for follow up and monitoring pediatric IBD patients. An important note is that OPG is more easily degraded at room temperature in the stool, and special preservation and storage conditions should be applied.
6. High Mobility Group Box Protein 1
High mobility group box protein 1 (HMGB1) is released by the immune cells during inflammation. Vitali et al. (2011) demonstrated that HMGB1 was significantly elevated in fecal and biopsy samples of children with IBD compared to healthy controls [
89]. Furthermore, fecal HMGB1 correlated with FC, even though the cell sources for the two proteins are different. HMGB1 also correlates with mucosal changes in children with remission and active disease (moderate correlation with simple endoscopic score for Crohn’s disease (SES-CD) and endoscopic Mayo subscore. However, HMGB1 levels and activity indices (Crohn’s disease activity index (CDAI) and a partial UC Mayo score) did not correlate.
Accordingly, it is suggested that fecal HMGB1 as a marker of subclinical gut inflammation and a novel biomarker of mucosal healing [
40], taking into account that it can be elevated in enteric infection as well [
41]. It was also sown in animal models that HMGB1 could be treated with dipotassium glycyrrhizate or ethyl pyruvate, which should be further investigated in human IBD [
82].
7. Chitinase 3-Like 1
Chitinase 3-like 1 (CHI3L1) expression in colonocytes and lamina propria macrophages is upregulated as a factor that facilitates bacterial penetration and adhesion to epithelial cells [
82]. This protein was found elevated not only in adults with IBD but also in children. Aomatsu et al. [
90] showed a marked increase of fecal CHI3L1 in children with IBD. Even a cut-off of 13.7 ng/g in fecal samples could differentiate between children’s IBD and controls with 84.7% sensitivity and 88.9% specificity. CHI3L1 levels were also shown to correlate with the scores for endoscopic severity of UC and CD, and with FC levels. The concentrations of the protein were dropped down in pediatric patients in remission.
Interestingly, CHI3L1 may be involved in neoplastic inflammation-related changes of the colonic epithelial cells in addition to his alleged role in IBD [
91]. This, mainly, should be investigated further since the chronic inflammation accompanying IBD affects children early in their lives and may increase the cancerogenic susceptibility.
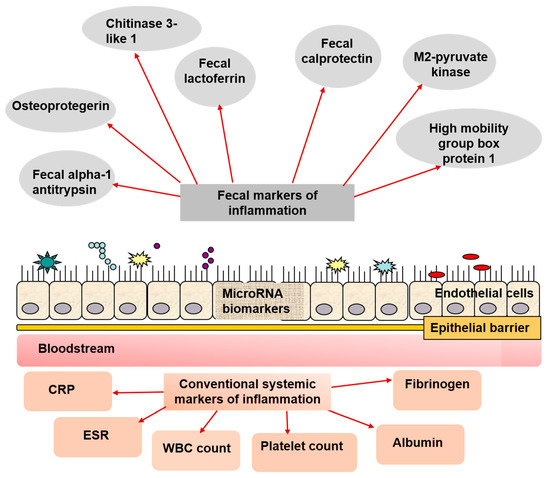
An overview of the fecal and serum biomarkers available for pediatric IBD is shown in .
Figure 1. Biomarkers employed in pediatric IBD diagnosing, managing, and follow-up. Along with the conventional non-specific markers for inflammation, there are a great variety of fecal biomarkers that can be easily assessed in fecal samples, and in blood. A promising perspective is assessing microRNA in the mucosa, which alterations can be attributed to IBD, including in the pediatric practice.
It is worth mentioning that the most recent guidelines of ESPGHAN, NASPGHAN, and ECCO recommend the routine use of laboratory parameters not only for the diagnosis [
92,
93] but also for the management of pediatric IBD [
94,
95,
96].
Blood tests (CBC, albumin, CRP, ESR, etc.) should be performed regularly by pediatric patients with UC depending on their symptoms and therapy and at least every three months while on immunosuppressive medications and at least every 6–12 months otherwise. Measurement of FC is advisable to verify mucosal healing in the patients in clinical remission, as a significant endoscopic activity may be present in 20% of children with PUCAI < 10. In addition, values of FC > 250 mg/g accurately predict mucosal inflammation and may select those patients who require endoscopic assessment and therapy escalation [
94,
95].
Regarding pediatric CD, repeat FC measurements in patients in clinical remission make it possible to identify a disease flare early, as FC increase precedes the disease relapse by 2–3 months. The combination of FC and CRP is superior in detecting endoscopic disease activity than FC alone. On the other side, the constellation of clinical remission, FC < 250 µg/g and CRP < 5 mg/L is the best non-invasive test for mucosal healing and can be used for treatment target [
96].
The available information regarding the performance of all the discussed systemic and fecal markers for detecting intestinal inflammation and disease activity is presented in , when applicable.
Table 1. Laboratory markers for the pediatric inflammatory bowel disease (IBD) population. Not all proposed biomarkers have validated data on the cut-off, sensitivity, and specificity for detecting intestinal inflammation and disease activity in the pediatric population.
This entry is adapted from the peer-reviewed paper 10.3390/gastroent11020009