Adiponectin is the adipokine associated with insulin sensitization, reducing liver gluconeogenesis, and increasing fatty acid oxidation and glucose uptake. Adiponectin is present in the kidneys, mainly in the arterial endothelium and smooth muscle cells, as well as in the capillary endothelium, and might be considered as a marker of many negative factors in chronic kidney disease. The last few years have brought a rising body of evidence that adiponectin is a multipotential protein with anti-inflammatory, metabolic, anti-atherogenic, and reactive oxygen species (ROS) protective actions. Similarly, adiponectin has shown many positive and direct actions in kidney diseases, and among many kidney cells. Data from large cross-sectional and cohort studies showed a positive correlation between serum adiponectin and mortality in chronic kidney disease. This suggests a complex interaction between local adiponectin action, comorbidities, and uremic milieu.
- adiponectin receptors,atherosclerosis,bone,diabetes
1. Introduction
Since its discovery, adiponectin has been identified as one of the key regulators involved in glucose and lipid metabolism. Further analyses have shown its anti-inflammatory and anti-apoptotic roles in human cells. It is produced predominantly in adipocytes. Human adiponectin protein has 244 amino acids (30 kDa), as well as the complex primary structure of a signal peptide, a hyper-variable region, a collagenous domain of 22 G-X-Y repeats, and a globular domain. It is present in human serum in relatively high concentrations, in three different structural forms: trimer (low molecular weight, LMW), hexamer (middle molecular weight, MMW), and 12–18-mer (high molecular weight, HMW). Another circulating, active form is globular adiponectin. It is generated by proteolytic cleavage of full-length adiponectin and has biological activity in humans [1]. Adiponectin concentrations are gender specific, with higher levels in females [2][3]. There are three known receptors for adiponectin: adiponectin receptor 1 (AdipoR1), adiponectin receptor 2 (AdipoR2), and T-cadherin. The first two receptors have a similar structure, with seven transmembrane domains, and an intracellular zinc binding motif capable of downstream signaling in the cell [3]. Novel research has also shown distinct ceramidase activity for the intracellular part of these receptors [4]. AdipoR1 is widely present in human cells, with the greatest numbers in skeletal muscle, AdipoR2 is mainly present in the human liver. Globular adiponectin has the highest affinity to AdipoR1, therefore in animal studies it acts mainly in muscle cells [1]. T-cadherin, lacking a transmembrane structure, is considered a binding protein for the high molecular weight (HMW) form. Some studies have indicated that T-cadherin is a major binding partner for adiponectin, and causes its accumulation in the heart, vascular endothelium, and skeletal muscle. The direct downstream effect of this binding is still under investigation [3][5][6]. Adiponectin may also bind to calreticulin, which is present on the surface of macrophages, and possibly other cells [7][8]. There are several factors regulating the expression of adiponectin gene (AdipoQ), such as: forkhead box O1 (FOXO1), peroxisome proliferator-activated receptor γ (PPAR-γ), CCAAAT-enhancer binding protein α, and sterol regulatory element binding protein 1c (SREBP-1c) [9]. Despite more than 20 years of constant studies on adiponectin function in animals and humans, an increasing quantity of data has been added recently. As an example, adiponectin might be one of the key mediators responsible for regular physical activity benefits in humans. Adiponectin connects energy balance regulation in the central nervous system and peripheral tissues [10]. Interestingly, animal studies have shown that adiponectin ameliorates the proinflammatory effect of saturated free fatty acid rich diet in hypothalamus. This is achieved mainly by suppression of microglia cell activation [11]. Other authors concluded that decreased serum adiponectin to leptin ratio is an indicator of adipose tissue inflammation and dysfunction and might predict cardiovascular risk in humans [12]. Adipocyte line studies have shown a direct HMW–adiponectin effect in reducing the local inflammation caused by glucolipotoxicity, via the APPL 1-AMPK pathway [13]. Adiponectin injection might also attenuate renal cell apoptosis in rats exposed to chronic intermittent hypoxia. In this study adiponectin reduced reactive oxygen species (ROS) generation and endoplasmic reticulum stress in the renal tissue [14]. It is a well-known fact that patients with chronic inflammatory states, such as rheumatoid arthritis or inflammatory bowel disease, have high serum adiponectin concentrations. This might be partly explained by a compensatory adiponectin expression, however animal and in vitro studies have shown that adiponectin might directly stimulate proinflammatory factor secretion in various cells, for example fibroblast-like synoviocytes, or neutrophiles of colonic lamina propria T-lymphocytes. Further studies differentiated the potential effect of different molecular forms of adiponectin in inflammation. Data suggest that, particularly HMW adiponectin and its globular form, contrary to low molecular weight (LMW) adiponectin, might also promote inflammation via dose-dependent NF-κB stimulation [15]. In the face of multipotential and complex adiponectin function, in this review we will be analyzing its role in chronic kidney disease and its complications.
2. Adiponectin Function
Adiponectin has traditionally been associated with insulin sensitization, reducing liver gluconeogenesis, and increasing fatty acid oxidation and glucose uptake. In the liver, it inhibits phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. In skeletal muscle, it promotes beta oxidation and lowers lipid accumulation via activation of 5′-AMP-activated protein kinase (AMPK). This action is mediated by adaptor protein, phosphotyrosine interacting with the PH domain, and leucine zipper 1 (APPL1). Another key metabolic pathway of adiponectin function is peroxisome proliferator-activated receptor α (PPAR-α) activation, which also increases fatty acid oxidation in both muscle and liver and increases glucose uptake in the latter organ. Generally, AdipoR1 activation triggers AMPK, while AdipoR2 activation triggers PPAR-α. Globular adiponectin has its unique effects on muscle metabolism and proliferation. Animal and cell line studies have shown an important adiponectin role in muscle differentiation and regeneration, and also as an autocrine/paracrine factor [10][16][17]. Additionally, adiponectin exerts an important protective role against ROS, by increasing oxidative stress detoxifying enzymes in mice skeletal muscles, mainly through the peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) pathway in mitochondria [18][19]. It has been shown that nonalcoholic fatty liver disease, chronic alcoholic fatty liver diseases, and chronic hepatitis C were associated with reduced serum adiponectin levels, decreased hepatic adiponectin receptor expression, and impaired hepatic adiponectin signaling [20].
Adiponectin also has a prominent anti-inflammatory function. It inhibits nuclear factor κB (NF-κB) signaling, tumor necrosis factor (TNF-α) secretion, and expression of adhesion molecules. Furthermore, it increases interleukin 10 (IL-10) and interleukin 1 receptor 4 (IL-1R4) production and promotes macrophage polarization into the anti-inflammatory M2 phenotype. Research on mice overexpressing adiponectin indicated a lower expression of other proinflammatory cytokines, including interleukin 12 (IL-12), interleukin 17B (IL-17B), and interleukin 21 (IL-21) in adipocytes and stromal vascular cells. There was also downregulation of cytokine expression, including intercellular adhesion molecule 1 (ICAM-1), C-C motif chemokine ligand 5 (CCL5/RANTES), granulocyte colony-stimulating factor (GCSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor receptor-1 (VEGFR-1), and thrombopoietin (TPO) in those cells [16][21]. Studies have also shown its anti-apoptotic role among various cells. Adiponectin attenuated oxidative stress caused by diabetes in cultured human umbilical vein endothelial cells through the cAMP/protein kinase A (PKA) pathway [22]. On the other hand, oxidative stress might block adiponectin secretion in murine adipocytes through PPAR-γ inhibition [9]. Another important aspect is the ceramidase activity of both AdipoR1 and AdipoR2 [3][23]. Analysis of these two receptors showed that, despite their difference in crystal structure, they are both capable of breaking down ceramide to sphingosine and free fatty acids [4]. After phosphorylation, sphingosine was turned into sphingosine 1-phosphate (S1P), an intracellular signaling molecule playing an independent part in insulin sensitization and metabolism in animal studies [24]. Serum adiponectin levels are significantly lower in many diseases, including lipodystrophy, type 2 diabetes (T2D), obesity, metabolic syndrome, and atherosclerosis. Higher than normal concentrations are observed in type 1 diabetes (T1D), and in diabetic nephropathy [9]. Adiponectin’s antiatherogenic properties have been proven in many studies. It inhibits smooth muscle cell proliferation, and decreases the expression of endothelial adhesion molecules, thus mitigating regional inflammation [25]. Adiponectin takes part in energy balance regulation, acting directly in the central nervous system. Animal studies have shown that hexameric and trimeric adiponectin forms can cross the brain–blood barrier, and that adiponectin receptors, AdipoR1 and AdipoR2, are present in the hypothalamus. Adiponectin activates AMPK in the hypothalamus and affects local proopiomelanocortin and neuropeptide Y signaling. Previous animal studies of adiponectin’s role brought conflicting results, showing anorexigenic or orexigenic effects [26][27]. Novel studies [28] have suggested that adiponectin action might depend on central nervous system glucose level. With high glucose concentration, adiponectin increased food intake through inhibition of proopiomelanocortin signaling. An opposite effect occurred with low glucose. Recent animal studies [29][30] have also shown adiponectin’s ability to relax gastric muscles, causing gastric distention, which might lead to early satiety and decrease food intake.
3. Adiponectin in Chronic Kidney Disease
In the last two decades, many trials have shown the emerging role of adiponectin in kidney disease. The main cause of morbidity in this population remains cardiovascular disease (CVD). Other complications include malnutrition, atherosclerosis, chronic inflammation, and elevated oxidative stress [31][32][33][34]. Despite a negative metabolic status, patients with end-stage renal disease (ESRD) have two to three times higher serum adiponectin levels than subjects with normal kidney function. This is partly due to increased adiponectin secretion from adipose tissue. Serum adiponectin in patients on either peritoneal dialysis or hemodialysis is approximately three-fold higher than in the general population, and none of those methods remove adiponectin significantly [35][36]. Some studies pointed out that factors contributing to lower adiponectin secretion are oxidative stress and sympathetic nervous activity, which are common in chronic kidney disease [35]. There is also a negative correlation between visceral fat, and adiponectin production and plasma concentration. Nagakawa et al. [37] also found a negative association between the number of metabolic syndrome components and plasma adiponectin. Marinez Cantarin et al. [38][39] demonstrated increased AdipoR1 expression in the skeletal muscles of uremic patients, and elevated AdipoR1 mRNA expression in their adipose tissue. Furthermore, there was increased AMPK phosphorylation in skeletal muscles, but its secondary downstream effects were impaired. Both acetyl-CoA carboxylase phosphorylation and carnitine palmitoyl transferase-1 concentrations were decreased in skeletal muscles, causing reduced fatty acid oxidation. Similar negative effects have been observed after incubation of human myocyte cultures in uremic plasma. In contrast, Sopić et al. [40] suggested a downregulation of AdipoR1 in peripheral blood mononuclear cells in hemodialysis patients. Although these results are partially conflicting, they all suggest a possible blocking of adiponectin action in chronic kidney disease (CKD) patients. There is also a significant growth of serum adiponectin levels in diabetic nephropathy, particularly in patients with A3 albuminuria and advanced stages of diabetic nephropathy. This chiefly contributes to elevation of the HMW form of adiponectin, whose concentration is inversely associated with estimated glomerular filtration rate (eGFR). Higher levels of adiponectin are an independent risk factor for severity of diabetic nephropathy. Animal models have shown a decrease of AdipoR density and function in the kidneys of diabetic rats, but the results were inconsistent, especially in the case of AdipoR2, and need further elucidation [9].
9. Summary
The last few years have brought a rising body of evidence that adiponectin is a multipotential protein, with anti-inflammatory, metabolic, anti-atherogenic, and ROS protective actions. These results, which have been acquired from numerous studies in predominantly animal or cell lines, have raised hopes for new therapies for medical conditions such as diabetes, atherosclerosis, or obesity. Similarly, adiponectin has shown many positive and direct actions in kidney diseases and among many kidney cells. In contrast, data from large cross-sectional and cohort studies showed a positive correlation between serum adiponectin and mortality in CKD. The nature of this adiponectin paradox is still unclear. Some authors suggest that increases in adiponectin might strictly be associated with lower renal clearance or might be a compensating mechanism related to the growing number of insults related to kidney function loss. On the other hand, some well-constructed studies have shown an independent correlation between adiponectin and mortality. Adiponectin is also strictly related to PEW, natriuretic peptides, and, in some trials, vascular calcifications, which are all distinct mortality risk factors for CKD. The causal relationship of adiponectin and these complications is still undetermined (Figure 1).
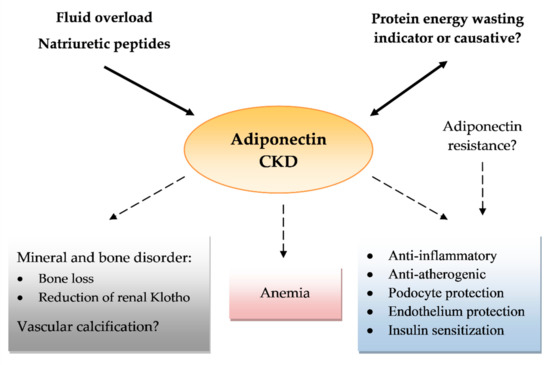
Figure 1. Effects of adiponectin in chronic kidney disease (CKD). Arrows pointing at adiponectin show a role of protein energy wasting, fluid overload, and natriuretic peptides in increasing serum adiponectin levels as a possible confounding factor. A number of question marks reflect the uncertain effects or conflicting results of reports.
The multidirectional involvement of adiponectin in the pathogenesis of kidney diseases is associated with a broad spectrum of challenges. Identifying mechanisms and pathways by which adiponectin is involved in the pathogenesis of kidney diseases is of particular interest and needs further studies. The search for biomarkers that predispose patients to a development of kidney diseases, and that may be helpful in monitoring of clinical course and their progression, will be the focus of research in the coming years. These biomarkers could include new molecules regulating T and B cell activation, proinflammatory chemokines, cytokines, and growth factors, as well as other proteins involved in immune response and fibrosis. The challenge is to develop appropriate strategies to investigate the molecular mechanisms involved in the pathogenesis of kidney diseases. The development of a certain algorithm including the molecular biomarkers could be helpful in the appropriate diagnosis and monitoring of kidney disease progression. Nevertheless, the search for these biomarkers will require further research.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249375
References
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321.
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100.
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109.
- Vasiliauskaité-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; De Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123.
- Kita, S.; Fukuda, S.; Maeda, N.; Shimomura, I. Native adiponectin in serum binds to mammalian cells expressing T-cadherin, but not AdipoRs or calreticulin. Elife 2019, 8, e48675.
- Clark, J.L.; Taylor, C.G.; Zahradka, P. Exploring the cardio-metabolic relevance of T-cadherin: A pleiotropic adiponectin receptor. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 200–206.
- Sun, Y.; Zhao, D.; Yang, Y.; Gao, C.; Zhang, X.; Ma, Z.; Jiang, S.; Zhao, L.; Chen, W.; Ren, K.; et al. Adiponectin exerts cardioprotection against ischemia/reperfusion injury partially via calreticulin mediated anti-apoptotic and anti-oxidative actions. Apoptosis 2017, 22, 108–117.
- Okada-Iwabu, M.; Iwabu, M.; Yamauchi, T.; Kadowaki, T. Structure and function analysis of adiponectin receptors toward development of novel antidiabetic agents promoting healthy longevity. Endocr. J. 2018, 65, 971–977.
- Zha, D.; Wu, X.; Gao, P. Adiponectin and its receptors in diabetic kidney disease: Molecular mechanisms and clinical potential. Endocrinology 2017, 158, 2022–2034.
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165.
- Lee, H.; Tu, T.H.; Park, B.S.; Yang, S.; Kim, J.G. Adiponectin reverses the hypothalamic microglial inflammation during short-term exposure to fat-rich diet. Int. J. Mol. Sci. 2019, 20, 5738.
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019, 11, 454.
- Pandey, G.K.; Vadivel, S.; Raghavan, S.; Mohan, V.; Balasubramanyam, M.; Gokulakrishnan, K. High molecular weight adiponectin reduces glucolipotoxicity-induced inflammation and improves lipid metabolism and insulin sensitivity via APPL1-AMPK-GLUT4 regulation in 3T3-L1 adipocytes. Atherosclerosis 2019, 288, 67–75.
- Ding, W.; Cai, Y.; Wang, W.; Ji, L.; Dong, Y.; Zhang, X.; Su, M.; Liu, J.; Lu, G.; Zhang, X. Adiponectin protects the kidney against chronic intermittent hypoxia-induced injury through inhibiting endoplasmic reticulum stress. Sleep Breath. 2016, 20, 1069–1074.
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219.
- Li, J.; Shen, X. Oxidative stress and adipokine levels were significantly correlated in diabetic patients with hyperglycemic crises. Diabetol. Metab. Syndr. 2019, 11, 13.
- Krause, M.P.; Milne, K.J.; Hawke, T.J. Adiponectin-consideration for its role in skeletal muscle health. Int. J. Mol. Sci. 2019, 20, 1528.
- Abou-Samra, M.; Selvais, C.M.; Dubuisson, N.; Brichard, S.M. Adiponectin and its mimics on skeletal muscle: Insulin sensitizers, fat burners, exercise mimickers, muscling pills … or everything together? Int. J. Mol. Sci. 2020, 21, 2620.
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319.
- Tsochatzis, E.; Papatheodoridis, G.V.; Archimandritis, A.J. The evolving role of leptin and adiponectin in chronic liver diseases. Am. J. Gastroenterol. 2006, 101, 2629–2640.
- Ge, Q.; Ryken, L.; Noel, L.; Maury, E.; Brichard, S.M. Adipokines identified as new downstream targets for adiponectin: Lessons from adiponectin-overexpressing or -deficient mice. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E326–E335.
- Ouedraogo, R.; Wu, X.; Xu, S.Q.; Motoshima, H.; Mahadev, K.; Hough, K.; Scalia, R.; Goldstein, B.J. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: Evidence for involvement of a cAMP signaling pathway. Diabetes 2006, 55, 1840–1846.
- Yanai, H.; Yoshida, H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: Mechanisms and perspectives. Int. J. Mol. Sci. 2019, 20, 1190.
- Reibe-Pal, S.; Febbraio, M.A. Adiponectin serenades ceramidase to improve metabolism. Mol. Metab. 2017, 6, 233–235.
- Lo, M.M.; Mitsnefes, M. Adiponectin, cardiovascular disease, chronic kidney disease: Emerging data on complex interactions. Pediatr. Nephrol. 2012, 27, 521–527.
- Klockars, A.; Levine, A.S.; Olszewski, P.K. Hypothalamic integration of the endocrine signaling related to food intake. Curr. Top. Behav. Neurosci. 2019, 43, 239–269.
- Wang, B.; Cheng, K.K. Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. Int. J. Mol. Sci. 2018, 19, 3552.
- Suyama, S.; Maekawa, F.; Maejima, Y.; Kubota, N.; Kadowaki, T.; Yada, T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci. Rep. 2016, 6, 30796.
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Can adiponectin have an additional effect on the regulation of food intake by inducing gastric motor changes? World, J. Gastroenterol. 2020, 26, 2472–2478.
- Idrizaj, E.; Garella, R.; Castellini, G.; Francini, F.; Ricca, V.; Baccari, M.C.; Squecco, R. Adiponectin decreases gastric smooth muscle cell excitability in mice. Front. Physiol. 2019, 10, 1000.
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081.
- Kovesdy, C.P.; Furth, S.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. Physiol. Int. 2017, 104, 1–14.
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as pxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752.
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671.
- Adamczak, M.; Chudek, J.; Wiecek, A. Adiponectin in patients with chronic kidney disease. Semin. Dial. 2009, 22, 391–395.
- Huang, J.W.; Yen, C.J.; Chiang, H.W.; Hung, K.Y.; Tsai, T.J.; Wu, K.D. Adiponectin in peritoneal dialysis patients: A comparison with hemodialysis patients and subjects with normal renal function. Am. J. Kidney Dis. 2004, 43, 1047–1055.
- Nakagawa, N.; Yao, N.; Hirayama, T.; Ishida, M.; Ishida, H.; Wada, A.; Fujino, T.; Saijo, Y.; Kikuchi, K.; Hasebe, N. Potential impact of renin-angiotensin system inhibitors and calcium channel blockers on plasma high-molecular-weight adiponectin levels in hemodialysis patients. Hypertens. Res. 2011, 34, 592–598.
- Martinez Cantarin, M.P.; Keith, S.W.; Waldman, S.A.; Falkner, B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol. Dial. Transplant. 2014, 29, 2268–2277.
- Martinez Cantarin, M.P.; Waldman, S.A.; Doria, C.; Frank, A.M.; Maley, W.R.; Ramirez, C.B.; Keith, S.W.; Falkner, B. The adipose tissue production of adiponectin is increased in end-stage renal disease. Kidney Int. 2013, 83, 487–494.
- Sopić, M.; Joksić, J.; Spasojević-Kalimanovska, V.; Bogavac-Stanojević, N.; Simić-Ogrizović, S.; Kravljača, M.; Jelić Ivanović, Z. Downregulation of AdipoR1 is associated with increased circulating adiponectin levels in Serbian chronic kidney disease patients. J. Med. Biochem. 2016, 35, 436–442.
