Adult neurogenesis, involving the generation of functional neurons from adult neural stem cells (NSCs), occurs constitutively in discrete brain regions such as hippocampus, sub-ventricular zone (SVZ) and hypothalamus. The intrinsic structural plasticity of the neurogenic process allows the adult brain to face the continuously changing external and internal environment and requires coordinated interplay between all cell types within the specialized microenvironment of the neurogenic niche. NSC-, neuronal- and glia-derived factors, originating locally, regulate the balance between quiescence and self-renewal of NSC, their differentiation programs and the survival and integration of newborn cells. Extracellular Vesicles (EVs) are emerging as important mediators of cell-to-cell communication, representing an efficient way to transfer the biologically active cargos (nucleic acids, proteins, lipids) by which they modulate the function of the recipient cells. At present, little is known on the physiological role of EVs in neurogenic niches.
- adult neurogenesis,extracellular vesicles,neural stem cell,neuron,astrocyte,microglia
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
It is now commonly accepted that discrete regions of the adult mammalian brain host neural stem cells that divide in situ and give rise to new neurons, a phenomenon referred to as “adult neurogenesis”.
The two most characterized neurogenic niches are the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), a brain region in which adult neurogenesis was confirmed in humans [1] and the subventricular zone (SVZ) of the lateral ventricles, whose relevance in adult human physiology is debated. Although SGZ and SVZ neural stem cells (NSCs) and neural progenitor cells (NPCs) share many features at cellular and molecular levels, the route for adult-born neuron integration in pre-existing neuronal circuits and their ultimate outcome in the two regions are distinctive. SVZ neurogenesis involves neuroblast migration along the rostral migratory system (RMS) to the olfactory bulb (OB), where they terminally differentiate into distinct types of olfactory neurons that are mainly inhibitory neurons [2]. In the last decades, pivotal preclinical studies, especially in rodents, have contributed to the idea that the integration of adult-born olfactory neurons facilitates continuous adaptation to environmental olfactory cues [3]. Conversely, mature granule neurons that originate from SGZ NSCs are excitatory neurons that are restricted to the granule cell layer (GCL), with minimal migration. At present, newborn DG neurons are considered to be crucially involved in specific types of hippocampal-dependent learning and memory, in stress and emotional responses [4,5]. Recently, adult neurogenesis has also been identified in the hypothalamus [6,7]. Here, tanycytes, which line the walls of the infundibular recess of the third ventricle, have been suggested as putative hypothalamic NSCs since they share the characteristics of SVZ and SGZ stem cells. In this region, adult-born neurons are regarded of crucial importance for the regulation of metabolism, energy balance [8] and systemic aging [9].
Regardless of the neurogenic region and the underlying complex functions, under physiological conditions, each step of adult neurogenesis needs to be tightly controlled by both niche-derived signals and by extrinsic environmental cues, which, together, ensure appropriate rates of NSC proliferation, differentiation, migration, neurite extension and integration of newborn cells into preexisting circuits [10,11]. This extensive modulation underlies the functional plasticity that is intrinsic to the neurogenic process, by which the brain outcome can be optimized for the needs of a given environment and/or experience.
It is generally accepted that a complete understanding of brain plasticity requires consideration of glial cells in the overall picture, and adult neurogenesis intriguingly connects neuronal and glial biology. Although all types of glial cells are directly or indirectly related to this process, astrocytes and microglia take on a prominent and active role. Astrocytes provide the closest link between adult neurogenesis and glial biology. In fact, several “astroglial” properties characterize NSCs in both neurogenic zones. The additional presence of essential non-neurogenic astrocytes within adult niches is also crucial for proper neurogenic process [12]. Astrocytes—which represent the most abundant cell type of the neurogenic niche—have been largely described as key regulators of the neurogenic process [12,13,14]. In the adult niche, astrocytes physically interact with NSCs [15,16,17] and with both developmentally and adult-born granule neurons [18]. In this context, they regulate NSC proliferation, differentiation and the functional integration of newborn neurons into the pre-existing network. Astrocyte communication with neurogenic niche cells also greatly depends on their paracrine activity. As one of the main secretory cells of the CNS [19], astrocytes release a myriad of gliotransmitters, neuromodulators and morphogens as well as metabolic, trophic and neuroprotective factors [13,14], by which they finely and positively regulate multiple steps of the neurogenic process. On the other hand, astrocytes can negatively modulate neurogenesis by both cell–cell contact and paracrine activity [17].
From being “silent” in healthy brain, microglia active role in adult neurogenesis has been profoundly reassessed in recent years. Evidence indicates that activated microglia plays a Janus-faced role in the context of adult neurogenesis, by favouring or counteracting NSC proliferation, differentiation and survival of adult-born neurons. These actions are mediated by both direct contact and paracrine mechanisms. For example, microglia have been shown to phagocyte newborn cells that undergo apoptotic death in SGZ and SVZ, thus ensuring the homeostasis of the neurogenic process [20,21]. In addition, microglia act as antigen-presenting cells interacting with peripherally derived immune cells. This interaction mainly occurs in the SVZ that is highly vascularized [22,23], thereby influencing NSC final commitment toward neuronal or glial phenotypes depending on the different kinds of activating T-cell stimuli (e.g., IL-4 or INF-y) [24]. On the other hand, microglia can influence adult neurogenesis through secretion of proneurogenic and/or antineurogenic molecules, whose balance determines the net outcome of adult-born neurons [25]. In particular, as the main driver of inflammatory processes in the brain, cytokines released by microglia can dramatically affect adult neurogenesis [26]. Altogether, astrocyte and microglia plasticity—which is reflected by their ability to acquire an anti- or pro-neurogenic phenotype—M1- and M2-states for microglia [27] and A1- and A2-states for astrocytes [28]—make these cells crucial actors in influencing NSC as well as responding to the complex and continuously changing neurogenic niche microenvironment.
An underestimated actor of the adult neurogenic niche is the neuronal component, which can participate in the regulation of neurogenesis dynamics. Recent evidence indeed suggests a bidirectional communication between developmentally and adult-born neurons [29,30]. Additionally, mature neurons were proposed to modulate adult neurogenesis by sending chemical signals to NSC [31].
2. Biogenesis and Function of Extracellular Vesicles
Extracellular Vesicles (EVs) are a heterogeneous population of membrane-bound entities that are released by both eukaryotic and prokaryotic cells [32] and that, by transporting different types of biomolecules, are key players in intercellular communication. Although much progress has been made in recent years in dissecting the molecular mechanisms underlying cargo packaging in recipient cells [33], further investigations are required to fully characterize the machineries and cellular pathways that determine the ultimate function (signaling or disposal) of cargo sorting in EVs.
The generation of EVs requires the fine-tuning of several intracellular molecular machineries and trafficking processes (as schematized in Figure 1). The best-characterized EVs are exosomes and microvesicles (MVs). Although the biogenesis of exosomes and EVs occurs at distinct sites within the cell, some common intracellular pathways and sorting machineries are involved in the generation of both types of EVs, thus hindering the possibility of discriminating between the different vesicle subpopulations [34].
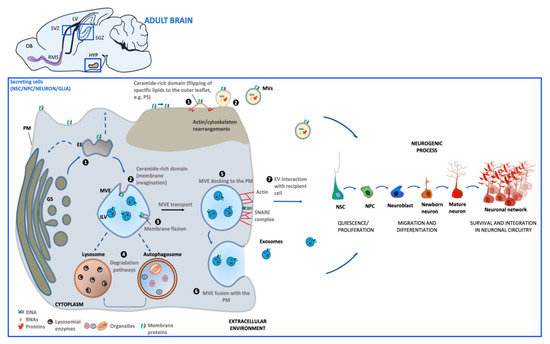
Exosomes (30–100 nm) derive from the endosomal compartment. Their formation starts with the generation of multivesicular endosomes (MVEs), spherical endosomes consisting of a limiting membrane and intraluminal vesicles (ILVs). The formation of MVEs is orchestrated by a complex of proteins called the endosomal sorting complex required for transport (ESCRT) which participates in the channeling of molecules into ILVs as well as the budding and fission of ILVs within MVEs [35]. However, there is evidence that exosome formation can also occur in a ESCRT-independent process [36]. MVE docking at the plasma membrane (PM) is regulated by RABs, actin and SNARE proteins, which finally promotes MVE fusion with PM and the release of the contained ILVs in the extracellular milieu as exosomes.
Microvesicles (MVs) (50–1000 nm) originate directly from PM by an outward budding which requires redistribution in lipid and protein composition and modifications in Ca2+ levels [37]. In MV biogenesis, Ca2+-dependent enzymes such as aminophospholipid translocases (flippases and floppases), scramblases and calpain drive the externalization of phosphatidylserine, which then drives changes in local membrane curvature and restructuring of the underlying actin cytoskeleton. These events are followed by the ATP-dependent fission process that leads to vesicle budding off from the PM and its subsequent release in the extracellular space [38,39].
Once released into the extracellular environment EV docking on target cell is regulated by specific interaction between membrane receptors on the recipient cell and EV enriched proteins. The uptake mode of EVs may be dependent on cell type, its physiological state as well as on the molecular composition at the PM of the target cell [40]. EVs can bound to the cell surface and initiate intracellular signaling pathways, be internalized or directly fuse with PM [41]. If internalized, EVs can fuse with the PM and release their contents into the cytoplasm of the recipient cell. Alternatively, EVs can target the endosomal pathway of the receiving cell and be directed toward the lysosome for the degradation of EV content to provide recipient cells with essential biological metabolites.
After interaction with target cells, EVs can elicit a variety of functional responses by delivering a wide array of biologically active molecules. These include lipids, proteins and nucleic acids, mRNA and other RNA species [(transfer RNA (tRNA), long non-coding RNA (lnRNA), micro RNA (miRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA)], which can be translated into proteins or regulate transcription in recipient cells, resulting in transient or persistent cellular phenotypic changes [42,43]. In particular, increasing evidence suggests that the effect of EVs on target cells is mainly dependent on the profile of intravesicular miRNA content [44]. By transferring miRNAs to target cells, EVs are now recognized as active players in intercellular gene regulation [45] because of their key natural roles in several cellular processes, including proliferation, differentiation, survival and apoptosis [46
EVs can support normal physiology by affecting stem cell maintenance [42], tissue repair [47], immune response [48], and blood coagulation [49], lipid metabolism [50], synaptic plasticity [51]. Under pathological situations, EVs can transport disease-associated proteins [52], thus contributing to propagate detrimental signals. Finally, since several molecular constituents in EVs have been found to be associated with specific diseases and treatment responses, EVs may represent reliable biomarkers which could serve as a diagnostic tool [53].
3. Extracellular Vesicles Generated in Adult Neurogenic Niches
The first publication of EVs released by neural cells was in 2004, when Février and colleagues demonstrated that glial cell lines overexpressing a prion protein released EVs that were capable of transferring infectivity in vitro and in vivo [54]. This work paved the way for the study of EVs as new tools exploited by neural cells for communicating with each other to guarantee normal brain function.
More recently, it has become increasingly evident that EVs may represent an additional key component of intercellular neurogenic niche communication. NSCs, neurons and glia have all been reported to release EVs that, in turn, can mediate a generalized cross-talk by niche components.
3.1. NSC-Derived Extracellular Vesicles
Endogenous adult NSC can generate EVs (NSC-EV).
A large array of studies have suggested that the exogenous administration of NSC-EVs in relevant animal models of acute and chronic neurodegeneration can foster neuroprotection and neuroplasticity [55,56,57,58,59]. Interestingly, these in vivo beneficial effects might largely depend on EV’s intrinsic properties that contribute to re-creating an immune-permissive environment that promotes brain repair and neurogenesis. Surprisingly, as of today, a much more limited number of studies have directly focused on the molecular/functional characterization and on the endogenous role of NSC-EVs on neuroplasticity and neurogenesis.
Based on this lack of knowledge, herein we reviewed the current direct and indirect knowledge of how endogenous NSC-EVs may affect and modulate different cellular components of the adult niche. Although more experimental efforts are required in this field, some interesting studies have opened the way to an initial understanding of the endogenous NSC-EV cargo and function. The different classes of pro-, anti-neurogenic and glia modulatory molecules found in EVs derived from NSCs are summarized in Table 1.
Table 1. List of different classes of pro-, anti-neurogenic and glia modulatory molecules found in extracellular vesicles derived from neural stem/progenitor cells-(NSC-EVs) and neurons (NDEs).
|
Class of Molecule |
Molecules |
Cellular Process/Molecular Target |
EV Type |
|
Growth factors |
Growth factor receptor cysteine-rich domain, EGF-like domain, EGF-like calcium-binding domain |
↑ NSC proliferation by activating the down-stream extracellular signal-regulated kinase (ERK) pathways [60] |
NSC-EVs [60] |
|
|
VEGF |
↑ NSC proliferation in SGZ [61]; ↑ survival and integration of newborn neurons in the forebrain [62] |
NSC-EVs [63] |
|
Proteins |
Flotillin, GAP43, Cadherin 2 L1CAM |
Regulate NSC proliferation and neuronal differentiation [64] |
NDEs [64] |
|
|
Cystatin C |
↑ NSC proliferation by cooperating with FGF-2 [65] |
NDEs [66] |
|
|
Ndfip1 |
↑ Removal of protein during stress [67] |
NDEs [67] |
|
|
Synaptotagmin 4 |
↑ Retrograde signaling in pre-synaptic cells by releasing Syt4-bound exosomes [68] |
NDEs [68] |
|
|
PRR7 |
↑ Removal of excitatory synapses by acting as a Wnt inhibitor [69] |
NDEs [69] |
|
|
MAP1b |
↑ synaptic transmission and plasticity [70] |
NDEs [71] |
|
Enzymes |
Asrgl1 |
↑ levels of aspartate/glutamate [72] which regulate adult neurogenesis [73,74] |
NSC-EVs [72] |
|
Cytokines |
INFγ |
Regulate function of microglia and astrocytes by activating Stat1 in target cells [75,76] |
NSC-EVs [77] |
|
miRNAs |
miR-21a |
↑ NSC proliferation by targeting Sox2 and Stat3 [78] |
NSC-EVs [78] |
|
|
miR-9 |
↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX [79] |
NSC-EVs [78] |
|
|
miR-let-7b |
↓ NSC proliferation and ↑ neural differentiation by targeting the stem cell regulator TLX and the cell cycle regulator cyclin D1 [80] |
NSC-EVs [78] |
|
|
miR-124 miR-137 |
Regulate NSC activation/proliferation, fate specification and differentiation by cooperatively targeting the pro-apoptotic protein BCL2L13 [81] |
NSC-EVs [82] |
|
|
miR-let-7 |
Regulate microglia activation which negatively affect NSC proliferation in SVZ [83] |
NSC-EVs [83] |
|
|
miR-9, miR-let-7, miR-26a, and miR-181c |
Regulate microglia morphology and physiology [84–87] |
NSC-EVs [83] |
|
|
miR-34a |
Regulate NSC proliferation and morphology and function of newborn neurons by interacting with DCX [88] Target genes linked to the regulation of neuronal excitability, mitochondria oxidative phosphorylation, glycolysis, and resting state functional connectivity [89] |
NDEs [89] |
|
|
miR-124 |
↑ NSC neuronal differentiation in SVZ [90] ↑ NSC neuronal differentiation in SVZ by targeting SOX9 [91] |
NDEs [92] |
|
|
miR-124-3p |
↑ GLT-1 expression in astrocytes [93] which ↑ NSC differentiation in vitro [94] and regulate synaptic transmission [95] |
NDEs [93] |
|
|
miR-21-5p |
↑ M1 polarization in microglia [96] |
NDEs [96] |
EGF: epidermal growth factor; VEGF: vascular endothelial growth factor; GAP43: growth-associated protein 43; L1CAM: L1 cell adhesion molecule; Ndfip1: Nedd4 family-interacting protein 1; MAP1b: microtubule -associated protein 1b; Proline-rich protein 7 (PRR7); Asrgl1: asparaginase-like protein 1; STAT1/3: signal transducer and activator of transcription 1/3; INFγ: interferon-γ. ↑: increased; ↓: decreased.
3.2. Neuron-Derived EVs in Neurogenic Niches
Neuron-derived EVs (NDEs) are increasingly gaining attention as a novel mechanism of cell-to-cell communication, including inter-neuronal crosstalk. Indeed NDEs can selectively bind to other neurons [108]. Based on these assumptions, within adult niches, NDEs can potentially contribute to modulation of neurogenesis by acting on NSC and/or their neuronal progeny directly or indirectly, via glial cells. Table 1 summarizes a list of pro-, anti-neurogenic and glia modulatory molecules associated with EVs of neuronal origin.
3.3. Glia-Derived Extracellular Vesicles
Although the current knowledge on the role of glial-derived EVs in adult neurogenic niches is limited, growing evidence suggest that these biological entities may be major players in the communication of astrocytes and microglia with NSC and their progeny (for reviews, see [120,121]). In the following paragraphs, we will discuss the potential role of EVs derived from astrocytes [Astrocyte-Derived Extracellular Vesicles (ADEs)] and microglia [(Microglia-Derived Extracellular vesicles (MDEs)] in regulating the adult neurogenic process. In particular, we posit that glia-derived EVs may have a prominent role in regulating the dynamics in the neurogenic zones, based on their presence in the ADEs and MDEs of biomolecules that have been functionally characterized as modulators of adult neurogenesis (Table 2).
Table 2. List of different classes of pro- or anti-neurogenic molecules found in astrocyte-derived (ADEs) and/or microglia-derived (MDEs) extracellular vesicles.
|
Class of Molecule |
Molecules |
Cellular Process/Molecular Target |
Glial EV Type |
|
Growth Factor |
FGF-2 |
↑ NSC proliferation and differentiation in SGZ and SVZ [122] |
ADEs [123] |
|
VEGF |
↑ NSC proliferation in SGZ [61]; ↑ survival and integration of newborn neurons in the forebrain [62] |
ADEs [123] |
|
|
Enzymes |
EAAT-1 |
↑ NSC differentiation, maturation and integration of newly formed neurons in synaptic network in SGZ and SVZ through regulation of extracellular glutamate [124] and GABA [125,126] levels |
ADEs [127] |
|
|
NTPDases |
↓ NSC proliferation in SGZ and SVZ by regulating nucleotide ATP and adenosine levels [128] ↓ NSC proliferation in hippocampus [129] and in vitro neuronal differentiation of SVZ NSCs [130] through adenosine production |
ADEs [131] |
|
|
CD13 |
↑ NSC proliferation, differentiation and survival through regulation of cAMP levels [132–135] |
MDEs [136] |
|
|
MCT-1 |
↑ NSC survival of newly generated neurons [137] |
MDEs [136] |
|
Neuroprotectant proteins |
Synapsins |
↑ NSC proliferation and survival in adult DG [138] ↑ synapse development [139], neurotransmitter release [140], neurite outgrowth after oxygen-glucose deprivation (OGD)/oxidative stress [141] |
ADEs [141] |
|
|
HSP70 |
↑ expression of genes involved in neuronal differentiation, synaptic activity, regulation of neuronal synaptic plasticity in Alzheimer’s disease [142] ↑ NSC proliferation, differentiation in DG via enhanced CREB phosphorylation and improve novel object recognition in mice [143] |
ADEs [144] |
|
|
Neuroglobin |
↑ NSC proliferation and differentiation in SVZ via Wnt signaling in murine stroke model [145] |
ADEs [146] |
|
Cytokines |
IL-1β |
↓ neurogenesis in DG by reducing the number of DCX+ cells [147] ↓ neurogenesis in DG by reducing the number of Nestin+ cells [148] ↓ hippocampal NSC proliferation in vitro via the nuclear factor-κB signaling pathway [149] ↑ NSC proliferation and differentiation through the activation of SAPK/JNK pathway [150] |
MDEs [151], ADEs [152] |
|
|
IL-6 |
↓ DG NSC proliferation in vitro [153] ↓ NSC proliferation, differentiation and survival in DG [154] ↑ NSC self-renewal and maintenance in SVZ [155] ↑ NSC proliferation and neuronal maturation in SVZ and SGZ [156] |
ADEs [157], MDEs [158] |
|
|
TNFα |
↑ NSC proliferation and survival through TNFR2 in vitro and in vivo [159] ↓ NSC proliferation and ↑ cell death through TNFR1 in vitro and in vivo [159,160] |
MDEs [158] |
|
miRNAs |
miR-302 |
↑ NSC proliferation, differentiation, survival through Cyclin D1/D2 and Fgf15 [161] |
ADEs [162] |
|
|
miR-let-7d, miR-let-7a |
↓ NSC proliferation and ↑ neural differentiation by targeting TLX receptor gene [163] ↑ NSC dopaminergic differentiation in olfactory bulb by PAX6 targeting (miR-let-7a,[164]) |
ADEs [163] |
|
|
miR-145 |
↑ NSC differentiation through Sox2-Lin28/let-7 signaling pathway [165] |
ADEs [163] |
|
|
miR-146a-5p |
↓ NSC neural specification and synaptogenesis by targeting neuroligin 1 (Nlg1) and synaptotagmin 1 (Syt1) [166] |
MDEs [167] |
|
|
miR-9 |
↓ NSC proliferation, ↑ NSC neural differentiation by targeting TLX receptor [79] |
ADEs [168] |
|
|
miR-9, miR-124 |
↑NSC neural differentiation and dendritic branching of differentiated neurons by targeting the small GTP-binding protein Rap2a [169] |
ADEs [168], MDEs [170] |
|
|
miR-184 |
↑ NSC proliferation, ↓ differentiation in SGZ by targeting Numblike [171] |
ADEs [162] |
|
|
miR-34a |
↑ NSC proliferation, ↓ dendrite branching and neuronal maturation by targeting DCX [88] |
ADEs [172], MDEs [167] |
|
|
miR-106b, miR-93, miR-25 |
↑ NSC proliferation and differentiation toward neuronal lineage in vitro through insulin/IGF-FoxO pathway [173] |
ADEs [162] |
FGF-2: fibroblast growth factor 2; VEGF: vascular endothelial growth factor; EAAT-1: excitatory amino acid transporter 1; NTPDases: nucleoside triphosphate diphosphohydrolases; CD13: aminopeptidase N; MCT-1: Monocarboxylate transporter 1; CREB: cAMP response element-binding protein; HSP70: heat shock protein 70; SAPK/JNK: stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK); TNFR1/2: tumor necrosis factor receptor 1/2; IL-1β: interleukin-1β; IL-6: interleukin-6: TNFα: tumor necrosis factor α. ↑: increased; ↓: decreased.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21228819
