Ventilation and gas exchange are fundamental to maintaining proper levels of oxygen (PaO₂) and carbon dioxide (PaCO₂) in the blood. Minute ventilation (V̇E), the total volume of air moved in and out of the lungs per minute, is normally around 5-8 L/min in healthy adults. However, only a portion of this air reaches the alveoli for gas exchange, a process known as effective alveolar ventilation (V̇A), which is crucial for regulating PaCO₂ levels. When effective ventilation is reduced or dead space in the lungs increases, carbon dioxide clearance decreases, leading to elevated PaCO₂, known as hypercapnic respiratory failure. Hypercapnic respiratory failure occurs due to two main mechanisms: decreased effective alveolar ventilation or increased dead space. Reduced ventilation may result from a lower respiratory rate or smaller tidal volumes, often caused by sedative drugs, brainstem injuries, neuromuscular weakness, or physical factors such as obesity. Alternatively, conditions like COPD or acute respiratory distress syndrome (ARDS) increase dead space, meaning more of the inhaled air does not participate in gas exchange due to inadequate blood perfusion in certain lung areas. This dead space effect prevents sufficient CO₂ elimination, even if minute ventilation is normal or elevated. Patients with chronic hypercapnia, such as those with advanced COPD, may tolerate higher levels of PaCO₂ (90-120 mm Hg) without symptoms. In contrast, healthy individuals typically begin to experience symptoms of hypercapnia when PaCO₂ rises above 70-80 mm Hg. On the oxygen side, the alveolar gas equation helps explain how PaO₂ depends on both inspired oxygen and the amount of carbon dioxide in the alveoli. Inadequate oxygen levels, or hypoxemia, can occur when effective ventilation is compromised, as seen in conditions that reduce alveolar ventilation or increase dead space.
- critical care medicine
- Hypercarbia
- Hypoxia
- Atmospheric Pressure
- Ventilation
- Oxygenation
To understand how hypercarbia (elevated PaCO₂) can lead to hypoxemia, here’s a breakdown using basic physiological principles and equations:
1. Alveolar Gas Equation for PaO₂
The alveolar oxygen partial pressure (PAO2P_{A\text{O}_2}) depends on inspired oxygen and carbon dioxide levels:
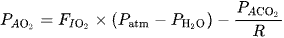
where:
- is the fraction of inspired oxygen.
- is atmospheric pressure (typically 760 mmHg at sea level).
- is the water vapor pressure at body temperature (47 mmHg).
- is the alveolar partial pressure of CO₂, which closely approximates PaCO₂.
- R is the respiratory quotient (typically ~0.8 for a normal diet).
2. How Hypercarbia Affects PaO₂
Increased PaCO₂ (hypercarbia) raises PACO2, reducing PAO2 because it displaces oxygen. This reduction in alveolar oxygen decreases the gradient for oxygen transfer to the blood, leading to lower PaO₂ (hypoxemia).
3. PaCO₂ and Ventilation
The arterial carbon dioxide level is related to alveolar ventilation:
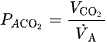
where:
- is the rate of CO₂ production.
- is alveolar ventilation, calculated as minute ventilation V˙A=V˙E−VD
If ventilation decreases, PaCO₂ rises, which in turn reduces PAO2 and PaO₂.
These equations demonstrate how hypercarbia from hypoventilation or increased CO₂ production leads to reduced oxygen levels in arterial blood.
In the context of respiratory physiology, hypercarbia (elevated CO₂ levels) and hypoxemia (reduced O₂ levels) are two critical manifestations of respiratory failure, each affecting patients differently depending on the underlying etiology and their respiratory capacity.
Hypercarbia
Hypercarbia, defined as an elevated arterial partial pressure of CO₂ (PaCO₂ >45 mm Hg), typically results from inadequate alveolar ventilation. It occurs when CO₂ production surpasses the lungs' ability to eliminate it, often due to reduced respiratory rate, decreased tidal volume, or increased dead space. Causes of decreased respiratory rate include sedative use, brainstem injuries, and central sleep apnea, while reduced tidal volume may stem from conditions like obesity, neuromuscular diseases, or chest wall deformities. In some cases, high dead space—where air is ventilated but not perfused adequately—contributes to elevated CO₂. This phenomenon is common in conditions like COPD and ARDS, where effective gas exchange is compromised despite adequate or even increased minute ventilationes, symptoms of hypercarbia include lethargy, confusion, and, at high levels, altered consciousness. Healthy individuals generally become symptomatic at PaCO₂ levels of 70-80 mm Hg, whereas those with chronic hypercapnia, such as patients with advanced COPD, may tolerate levels as high as 90-120 mm Hg without symptoms . When hyperca failure occurs, it often necessitates medical intervention, such as non-invasive positive pressure ventilation (NIPPV) or intubation, to enhance alveolar ventilation and improve CO₂ clearance.
Hypoxemia
Hypoxemia, defined as a PaO₂ <60 mm Hg, results from various mechanisms, including hypoventilation, diffusion impairment, shunt, and ventilation-perfusion (V/Q) mismatch. Each mechanism can present differently on arterial blood gas analysis and requires targeted treatment.
- Hypoventilation leads to reduced oxygen intake, often due to similar factors that cause hypercarbia, such as drug-induced respiratory depression or neuromuscular weakness.
- Diffusion impairment occurs when the alveolar-capillary membrane is thickened or damaged, as seen in interstitial lung diseases, making it harder for oxygen to enter the bloodstream.
- Shunt, a severe form of V/Q mismatch, involves blood bypassing the ventilated alveoli without gas exchange. This can occur in ARDS, severe pneumonia, or large lung consolidations, and is typically resistant to oxygen therapy.
- V/Q mismatch itself, often seen in obstructive or restrictive lung disease, results from poorly ventilated but well-perfused alveoli, leading to localized hypoxemia that responds to supplemental oxygen .
Relationship Between ypercarbia and hypoxemia can co-occur, particularly in conditions with severe hypoventilation or high dead space. For example, in COPD or ARDS, compromised alveolar ventilation due to airway collapse or dead space increases PaCO₂ while simultaneously reducing PaO₂. This dual deficit emphasizes the importance of managing both ventilation and oxygenation to prevent respiratory acidosis and tissue hypoxia.
Management Considerations
- Ventilated Patients: For mechanically ventilated patients, adjustments in positive end-expiratory pressure (PEEP), inspiratory time, and FiO₂ can help optimize oxygenation and ventilation. Humidification of inspired gases can reduce insensible water loss, especially critical in patients with elevated minute ventilation.
- Non-Invasive Strategies: In cases of mild hypercapnia or hypoxemia, NIPPV or high-flow nasal cannula may suffice to augment alveolar ventilation and improve oxygenation, potentially preventing intubation in patients at risk for respiratory failure .
Effective management of hypercarbia and hypoxemia requssessment of each patient’s ventilation-perfusion status, response to oxygen therapy, and any underlying factors contributing to respiratory compromise. By addressing both CO₂ clearance and O₂ supplementation, clinicians can mitigate the risks associated with respiratory failure and improve patient outcomes.
1. What is hypercarbia, and what are its main causes?
Answer: Hypercarbia is an elevated arterial partial pressure of CO₂ (PaCO₂ >45 mm Hg), primarily caused by inadequate alveolar ventilation. It results from factors such as decreased respiratory rate (e.g., due to sedatives or brainstem injuries), reduced tidal volume (e.g., in obesity or neuromuscular disease), or increased dead space (e.g., in COPD or ARDS). In these cases, CO₂ production surpasses the lungs' ability to eliminate it.
2. At what PaCO₂ levels do symptoms of hypercarbia generally appear in healthy vs. chronic cases?
Answer: Healthy individuals typically experience symptoms of hypercarbia, like confusion and lethargy, at PaCO₂ levels of 70-80 mm Hg. However, patients with chronic hypercapnia, such as those with advanced COPD, may tolerate PaCO₂ levels as high as 90-120 mm Hg before symptoms manifest.
3. What are the primary mechanisms that lead to hypoxemia?
Answer: Hypoxemia, defined as a PaO₂ <60 mm Hg, can result from several mechanisms:
- Hypoventilation (e.g., due to drugs or neuromuscular weakness)
- Diffusion impairment (e.g., interstitial lung disease)
- Shunt (e.g., ARDS or severe pneumonia, where blood bypasses ventilated alveoli)
- Ventilation-perfusion (V/Q) mismatch (e.g., obstructive or restrictive lung disease)
4. How do shunt and V/Q mismatch differ in terms of response to oxygen therapy?
Answer: A shunt occurs when blood bypasses ventilated alveoli entirely, resulting in hypoxemia that typically does not improve with supplemental oxygen. In contrast, V/Q mismatch involves poorly ventilated but well-perfused alveoli, leading to hypoxemia that generally responds to oxygen therapy.
5. How can hypercarbia and hypoxemia occur simultaneously, and in which conditions is this most common?
Answer: Hypercarbia and hypoxemia can co-occur when there is severe hypoventilation or increased dead space. This is commonly seen in conditions like COPD and ARDS, where compromised alveolar ventilation leads to both elevated PaCO₂ and reduced PaO₂. In these cases, both ventilation and oxygenation must be managed to prevent respiratory acidosis and tissue hypoxia.
6. What are the main strategies for managing hypercarbia and hypoxemia in mechanically ventilated patients?
Answer: In mechanically ventilated patients, management of hypercarbia and hypoxemia includes adjusting parameters like positive end-expiratory pressure (PEEP), inspiratory time, and fraction of inspired oxygen (FiO₂) to optimize both oxygenation and ventilation. Additionally, humidification of inspired gases can minimize insensible water loss, which is particularly beneficial in patients with high minute ventilation.
7. How can non-invasive ventilation strategies help manage mild cases of hypercarbia and hypoxemia?
Answer: In mild cases, non-invasive positive pressure ventilation (NIPPV) or high-flow nasal cannula (HFNC) can augment alveolar ventilation and improve oxygenation. These non-invasive options can help prevent intubation and reduce the need for mechanical ventilation in patients at risk for respiratory failure.
8. What clinical assessments are essential when managing patients with hypercarbia and hypoxemia?
Answer: Effective management requires assessing the patient’s ventilation-perfusion status, response to oxygen therapy, and underlying factors contributing to respiratory compromise. Monitoring parameters such as PaCO₂, PaO₂, blood pressure, and central venous pressure (CVP) is crucial for tailoring treatment to each patient’s needs.
