1. COVID-19 in Africa
Africa is the second most populated continent, with around 1.3 billion people (17% of the world population) [
1]. It comprises 54 independent nations, many of which are referred to as fragile states [
2,
3]. Original predictions indicated that Africa would be disproportionately affected by COVID-19 [
4], also in view of the difficulty of implementing effective measures of social distancing [
5]. The first case of COVID-19 was reported in Egypt on 14 February 2020 [
6]. Then, imported cases started to be detected in other African nations, as most Europeans were trying to escape the dramatic reality they were experiencing [
7]. African governments were highly sensitized to seeing countries with large economies being defeated by the virus and started to close borders and isolate nations from foreign entry [
7]. Although in Africa local transmission increased slowly [
7], the limited financial and qualified human resources to overcome the deadly disease forced this continent to preventively lock down [
5,
8]. The youthfulness of the African population is the most plausible explanation for reduced severity of COVID-19 in this continent. The average age is 19.7 years, and 60% of the population is younger than 25. Severe symptoms predominantly affected the elderly population [
9], who suffered from sub-chronic impairments of the immune system. In Africa, immunity is linked to multiple pathogen exposures [
10], which shape the immune response. This continuous activation is capable of reprogramming immune cells [
11] and providing them with the ability to respond differently to microbes [
12]. This is an advantage which allows individuals to better cope with infection, including during childhood. In the context of COVID-19, this notion was demonstrated by comparing the immunity elicited upon infection in Belgian, Canadian, Ecuadorian, and South African cohorts, the latter showing a reduced functional response of immune cells. Lifestyle trends, scarce consumption of processed food, climate, and environmental aspects are other factors that might have contributed to mitigating the dramatic effects caused by the pandemic [
13]. This notion was supported by evidence showing that environmental parameters were able to contradict initial predictions and reduce virus lifespan [
14,
15]. While the low temperatures and humidity of the northern globe hemisphere facilitated the virus’ survival [
16], the warm African climate was shown to reduce the risk of COVID-19 infection [
17]. The effect of sunshine UV rays acted synergistically, on one hand compromising viral stability and diminishing disease transmission, and on the other causing a beneficial effect on the host. Individuals presenting an increased level of vitamin D were able to elicit a more efficient activation of an immune response against the virus [
18]. It is also worth mentioning that the windy weather in some countries was also considered an advantage. It negatively affected viral spreading [
19] and contributed to dispersing viral pathogens, lowering disease transmission. Hence, the growth curve for COVID-19 in Africa was inversely correlated to average temperature and wind speed [
20].
However, a shift from this initial positive scenario occurred when multiple SARS-CoV-2 variants started to appear. By the end of 2020, most African countries underwent a second wave of infection, with a 30% growth in weekly incidence and daily average of new cases, in relation to the peak of the first COVID-19 wave [
21]. Controversial suggestions were publicly advanced, with people proposing on international television channels to let the continent reaching heard immunity, a possibility that was finally not considered. Whether this was due to a global voice raised against those statements or to the fear of a potential “boomerang effect” is still not clear. Indeed, the many efforts made to contain the COVID-19 pandemic could have been jeopardized with a reestablished mobility between continents. The concern that implemented measures might have been of no use is possibly the reason for which “common sense” prevailed.
In March 2023, nearly 180,000 fatalities were reported in Africa vs. almost 8 million deaths registered worldwide. According to the WHO, South Africa and Morocco were the most affected [
3,
22]. In this regard, it should be highlighted that over 50% of African citizens reside in rural areas, with limited interaction with travelers or urban communities that could potentially carry the virus [
23]. When compared to other continents, the rate of foreign travel to Africa is relatively low. South Africa and Morocco have the highest rates, ranking, respectively, 22nd and 28th worldwide. This may explain why these countries showed the highest number of cumulative COVID-19 cases in Africa [
24].
Concerns regarding a possible discrepancy between predicted and confirmed cases stood out. The reduced morbidity and mortality registered in the African continent was inconsistent with the results obtained from seroprevalence tests [
25]. Antibodies were detected in 10–30% of the African population. However, this percentage also raised numerous discussions, as positivity relied on the testing capacity of the country where the assay was performed [
8]. No nations had the financial resources to purchase tests for all suspected cases. So, while there were countries who received more assistance than others from state cooperation and international donations, the percentage of conducted tests was not comparable to the developed world. While South Africa conducted a total of 54,224 tests per million people, Egypt and Nigeria carried out 1317 and 1504 tests, respectively. In the UK, the number of conducted tests was 266,500 per million people, and in the US it was 195,072 during the same period [
26].
However, despite the overall lower number of individuals that were reported to have developed severe symptoms [
4], COVID-19 in Africa is still a threat. The precarious situation, characterized by a variety of infectious diseases, malnutrition, and limited access to healthcare, has worsened since the pandemic occurred [
27,
28]. In addition, the inequality of vaccine distribution as well as of population adhesion to vaccination programs hindered African response to COVID-19 [
29].
2. Genetic Variants of SARS-CoV-2 Reported in Africa
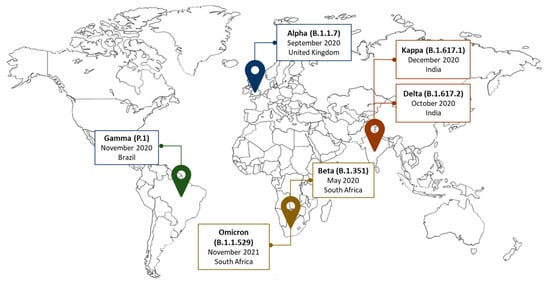
Virus variants emerged since the outbreak and mostly affected the spike protein, which became the target of vaccine and monoclonal antibody production (
Figure 1) [
48,
49]. The first variant was known as D614G. It appeared in April 2020, replacing the original SARS-CoV-2 by June 2020 [
48,
50]. A few months later, another variant was reported in the UK and was named Alpha (B.1.1.7) [
50]. Many additional mutations occurred since then, showing distinct effects, in terms of infection spread and symptom severity, related to the affected region. In the spike protein, mutations like N501Y increased virus transmissibility [
48]. Similar effects were also identified in variants affecting the non-spike and nucleocapsid regions, resulting in different sub-lineages [
50]. South Africa was the incubator of the Beta variant (B.1.351) [
50], a highly transmissible strain, sharing D614G and N501Y mutations with the Alpha variant. Unexpectedly, B.1.351 was less effectively neutralized by conventional therapies [
48]. While the country was severely affected, Brazil reported a new variant, the Gamma (P.1) variant [
51], causing an infection rate of approximately 75%. Mutations in the spike protein region [
48,
50] and in the amino-terminal domain [
50] characterized P.1. This variant was not reported in Africa, raising concerns regarding how much sequencing the continent could afford. Nevertheless, the Delta variant (B.1.617.2) started to spread. From India, it reached Africa, where it significantly increased the morbidity and mortality rate of many fragile nations [
3,
52]. The same occurred with the Kappa (B.1.617.1) variant, although its transmissibility and number of sub-lineages were lower [
50]. Despite all these mutations, the variant that prevailed in Africa was Omicron (B.1.1.529) [
3,
52,
53,
54]. The many genetic changes affecting Omicron [
55] increased its infectivity [
56], but reduced the risk of the virus causing severe clinical outcomes. Hence, this variant became less lethal [
57]. Since then, different mutations were identified in Omicron and many sub-variants were reported [
58,
59,
60]. Some emerged as lineage combinations, and their evolution is still closely followed.
Figure 1. Variants of concern. RNA viruses, such as SARS-CoV-2, constantly evolve. Some mutations can provide the virus with a selective advantage, like increased transmissibility.
3. The Transmissibility of SARS-CoV-2 and the Difficulty of Applying Standardized Protection Measures in Africa
The fear of unprecedented mortality in Africa due to the COVID-19 pandemic grew exponentially when it was realized that the best healthcare provided in the developed world was not sufficient to stop the lethality of SARS-CoV-2. The virus was shown to replicate from 5 to 11 days [
61,
62]. Then, common symptoms, like fever, fatigue, and dry cough, began to appear [
63]. Malaise and myalgias were also reported [
64], as well as headache, abdominal pain, diarrhea, nausea, and vomiting [
65]. While anosmia and ageusia were considered early markers of COVID-19 [
64], shortness of breath was suggestive of a worsening disease stage [
65]. Hence, protection measures to mitigate the infection started to be standardized. However, soon after their implementation, it became clear that they were designed for different realities than that in Africa. The virus was transmitted through close contact with infected individuals and the inhalation of viral-containing particles in the form of microscopic aerosols [
66,
67,
68,
69,
70,
71,
72,
73]. Thus, how could distance be maintained among people that mostly live of the informal sector or running small restoration businesses? How could home confinement be followed by people belonging to economically devasted communities, in which street selling remains the only source of sustenance? The difficulty of adopting standardized norms also referred to preventive measures of hygiene. Besides alcohol shortages, inequalities were highlighted even upon recommendations of simple actions, such as hand washing. In Africa, only one third of the population has regular access to clean water [
74]. So, not stressing measures that required financial support to be implemented, many took for granted the privileges of living in developed countries. When scientific evidence found active viruses in biological samples, like urine [
75,
76] and feces [
75,
77,
78,
79], even after patient recovery [
77,
80], many concerns were raised in the continent, as most African citizens do not have access to private and clean sanitary facilities. Hence, viral infectivity was also expected to significantly grow because of open drains and squat toilets, lacking flushing water systems [
81]. Accordingly, an enhanced number of cases started to be reported in countries where open defecation was a common practice. The lack of proper infrastructures for human waste management was seen as an additional risk for viral spreading [
82]. This emphasized that despite the effort made to educate society on how to avoid infection, the applicability of many measures caused a double-edge feeling. The rules globally adopted contradicted many socioeconomic and cultural aspects associated with an increased risk of viral transmission, such as breastfeeding, which was referred as one of the routes for SARS-CoV-2 transmission [
83]. In Africa, approximately 40% of infants are exclusively breastfed [
84,
85], a percentage below the WHO’s Global Nutrition Target of a 50% prevalence to be reached in 2025 [
86]. The hypothesis that those women could have turned into disease incubators stood out. Concerns were also expressed in the case of intrauterine spreading [
87], as the impact of COVID-19 became more evident in reproductive healthcare. In over 115 evaluated countries, including almost 50 African nations, maternal and child mortality rates increased monthly by 38.4% and 44%, respectively [
88]. Nowadays, since COVID-19 does not pose a deadly threat to the world anymore, efforts should be made to evaluate Africa’s response to viral infectivity, teaching lessons for future outbreaks and promoting policies to leverage healthcare.
4. The Comorbidities Underlying COVID-19 Severity in Africa
Among the comorbidities enhancing the severity of COVID-19, non-communicable diseases were recognized worldwide as a prevalent risk factor for poor clinical outcomes. Their incidence in Africa is a major concern [
52,
96]. Non-communicable diseases are expected to be the primary cause of mortality in African nations by 2030 [
97]. The Democratic Republic of the Congo, Nigeria, Ethiopia, and South Africa present the highest rates, considered as a potential explanation for the increased number of reported severe COVID-19 cases. According to the Global Burden of Disease studies, the incidence of non-communicable diseases in Africa has also been linked to the growing adoption of the Western lifestyle [
98,
99,
100].
Among those pathologies, diabetes mellitus stood out [
101]. In Africa, the number of diabetic individuals is 24 million and is projected to increase by 129% in 2045, reaching 55 million people [
102]. Diabetic patients were more likely to manifest severe COVID-19 symptoms. Their compromised immune system, hypercoagulable pro-thrombotic state, endothelial dysfunction, and vascular inflammation [
103] led to diabetes mellitus being a major contributor to COVID-19-induced complications and mortality [
104,
105,
106,
107]. According to WHO, during pandemic times, the fatality rate of diabetic patients was 10.2%, as assessed in 13 sub-Saharan countries. Conversely, in non-pandemic times, it was 2.5% [
108].
Another risk factor for the appearance of severe COVID-19 symptoms was cardiovascular diseases [
109]. Their prevalence varies from 0.1% in Sudan, where coronary events prevail, to 20% in Mozambique, where individuals mainly develop endomyocardial fibrosis [
97]. Childhood rheumatic heart disease is also widespread in sub-Saharan Africa [
110], ranging from 0.2% to 3% [
111,
112,
113,
114,
115]. In terms of long-term sequelae, the occurrence of thrombosis and cardiorenal syndrome, upon COVID-19 infection, prevailed [
94]. However, cardiovascular pathologies are also triggered by other highly prevalent infections, among which are HIV and tuberculosis [
116], alerting of the difficulty in discriminating cardiovascular impairments caused by SARS-CoV-2 or other pathogens. In Africa, almost 50% of the individuals that suffer from tuberculosis show symptoms of pericarditis [
97]. Its severity was significantly worsened by COVID-19 when individuals also carried HIV [
117]. Similar results were even obtained in relation to the number of casualties [
118]. Inflammation, elicited by SARS-CoV-2, exacerbated heart and blood vessel damage, as evidenced by the tropism of the virus for these cells [
119,
120]. Also, fibrosis-altered lung function and chronic obstructive pulmonary disease were reported as long-term COVID-19-induced sequelae in patients with tuberculosis [
94,
121,
122]. Coinfections are prevalent in Africa. In the presence of different pathogens, the response to COVID-19 was impaired [
52], as revealed in a study including 17,871 participants infected with SARS-CoV-2 [
123].
Another concern drawing attention to severe COVID-19 symptomatology was obesity. According to WHO, 20% of adults and 10% of children and teenagers in South Africa, Nigeria, Mozambique, Uganda, Tanzania, Zambia, Zimbabwe, Kenya, Malawi, and Ethiopia, were expected to be classified as obese by December 2023 [
124]. Although a causal relationship between obesity and COVID-19 was not fully established, evidence suggested they are strictly related. Hospitalized COVID-19 patients manifesting adult respiratory distress syndrome were often obese [
125,
126,
127,
128] and presented associated comorbidities [
129]. However, differences in obesity epidemiology might have impacted the severity of COVID-19, especially when referring to urban regions. Most African people living in rural areas suffer from malnutrition, which is known to impair immune response and increase symptom severity [
130]. In Angola, Liberia, Tanzania, Burkina Faso, Chad, Mali, Niger, Sudan, as well as in the Northern African region and Yemen, malnutrition and iron deficiency prevail, presumably justifying the high mortality caused by COVID-19 [
131].
The prevalence of diseases related to disruption of iron metabolism also highlighted the possibility that deficiency of this metal might have played an important role in the fight against COVID-19 in Africa. Iron is a vital element, ensuring the development and survival of many organisms [
132]. Viruses also need iron to proliferate, as it is required for the synthesis of their genetic material and energy production. Despite the lack of studies evaluating the correlation between iron levels and COVID-19 severity in Africa, numerous investigations were conducted in developed countries. In human volunteers, the exposure to norovirus was associated with the induction of hypoferremia, caused by an increased hepcidin, the hormone regulating iron absorption and circulating levels [
133]. Dysregulation of iron homeostasis in COVID-19 patients was observed since the beginning of the outbreak [
134]. The analyses of 99 patients, hospitalized in Wuhan Jinyintan Hospital with COVID-19-induced pneumonia, showed tissue iron overload and increased serum ferritin, the neutralizing protein storing iron within its subunits [
135]. A higher production of pro-inflammatory cytokines directly linked to iron levels, like IL-1, IL-6, INF-γ, and TNF, was associated with a poor prognosis [
136,
137]. The cross-talk between inflammation and iron metabolism led researchers to investigate if hepcidin levels could predict the fatal outcome of COVID-19 infection [
138]. Patients with hepcidin levels below 394 ng/mL were found to be more likely to survive. Higher levels of hepcidin were shown to increase inflammatory markers, like CRP and ferritin, as assessed in a descriptive study conducted with 5700 COVID-19 patients [
127]. Hypoferremia was manifested in almost all ICU-admitted COVID-19 patients [
139] and was associated with expression of organ damage markers, like aspartate aminotransferase-AST and lactic acid dehydrogenase-LDH [
140,
141]. Severe COVID-19 was also linked to low levels of hemoglobin, possibly as a consequence of the lower iron levels available for its synthesis. This negatively impacted erythrocyte production, as confirmed by the decrease in erythropoietin [
142]. Its release also depends on the ability to sense hypoxia [
141,
143], and patients suffering from severe hypoxemia were found with lower levels of serum iron [
144]. Given that the activation of immune cells relies on iron [
143], low levels of this metal justified the impaired T cell response reported in COVID-19 patients [
144,
151].
5. Vaccine Inequity Distribution in Africa
Contrarily to the multiple vaccination rounds that developed countries benefited from, most African nations did not reach complete vaccination coverage (
Figure 2) [
216]. Several aspects contributed to the vaccine distribution inequity, including the poor social services taking care of vaccine storage, the conflicts and wars occurring in different countries, the internal migration issues and environmental degradation that hindered vaccine transportation, and a weak governance, violating human rights [
3]. Many African countries also lacked the logistics necessary to receive and administer vaccines against SARS-CoV-2, some of which required low-temperature storage and efficient energy and power to maintain freezer functioning. The level of medical preparedness was a challenge. Contrarily to developed countries, the number of health care professionals able to plan, supervise, and follow vaccination procedures was low [
3]. This hindered training new nurses and physicians, as the scarce qualified personnel were occupied with saving lives. Insufficient investment, even related to basic equipment and hospital beds, increased the vulnerability of Africa to COVID-19. However, the assumption that most SARS-CoV-2 infections in Africa were asymptomatic or associated with mild clinical symptoms contributed to the decision to regard the continent as a non-priority [
216]. The disparity in vaccine distribution, as shown in the figure below, reflected the economic power of many countries and should have raised ethical concerns.
In Africa, vaccination programs began on 1 March 2021. The COVAX Facility, co-led by the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi, the Vaccine Alliance, and WHO, secured around 2 billion doses. Vaccines were distributed by the end of the year [
218,
219], through the cooperation of the Africa Centers for Disease Control and Prevention, the African Union, and the WHO Regional Office for Africa. One of the biggest successes of this partnership was the acquisition of 400 million doses of the Johnson & Johnson single-dose vaccine before the end of 2022 [
216]. The AstraZeneca vaccine was approved on 15 February 2021 [
220] and had great repercussions in the continent due to its cost-effectiveness, ease of storage, and transportation [
3]. It is therefore fair to highlight that it contributed to increasing the vaccination rate in Africa. The International Rescue Committee (IRC) estimated that the extra doses in the US, UK, and EU could have vaccinated individuals over 16 years old in the 20 countries most at risk of humanitarian disaster [
221]. However, the multiple waves of COVID-19 reduced donations and caused vaccine shortage. On 19 March 2023, the African continent registered over 55 COVID-19 vaccine doses administered per 100 people, which was significantly lower when compared to the global average of over 167 doses per 100 people [
222]. According to WHO, more than 1 billion doses have been administered in Africa, with 61% of them coming from COVAX, 27% from bilateral agreements, and 11% from the African Union’s African Vaccine Acquisition Trust (AVAT). The COVID-19 vaccines used in Africa include Janssen (35%), Pfizer/BioNTech (19%), AstraZeneca (15%), Sinopharm (13%), Sinovac (7%), Moderna (5%), and others (7%) [
223]. So far, the African continent has achieved 36.7% coverage of COVID-19 vaccination among its population, which is significantly lower than the global coverage of 69.7% [
222,
223]. Sierra Leone and Botswana showed the highest level of immunization, reaching 96%, followed by Tunisia (94%) and Eswatini (93%). Conversely, Burundi, Namibia, and the Republic of Congo presented the lowest vaccination coverage, with 4%, 25%, and 26%, respectively. Eritrea is yet to start its COVID-19 vaccination campaign [
223], as vaccine hesitancy in sub-Saharan Africa slowed the inoculation rate of COVID-19 vaccines. Some countries refused to participate in the COVAX free vaccine program or delayed vaccination programs due to anti-vaccine sentiments and religious beliefs [
224]. Some donated vaccines with a short shelf life rapidly expired [
225,
226]. Yet, Africa is still vulnerable to new and more transmissible variants of SARS-CoV-2 that may require country-specific solutions [
13,
221]. The need for African countries to invest in medical science, so as to reduce scientific dependence and assistance from developed nations [
3], led several agreements to share vaccine technology, as proposed by Moderna, WHO, BioNTech, and Afrigen Biologics and Vaccines in South Africa [
227,
228]. The manufacturing capacity was already assessed in Tunisia, Senegal, Egypt, Ethiopia, and South Africa. However, commercial capabilities require further development, given the need to reinforce business planning and market potential, since products produced in many African countries are not accepted in developed regions, as experienced by Rwanda. Whether lucrative markets and parallel importation may be the cause of competing conflicts, to overcome healthcare inequality, multilateral collaborations are required. Sustainable and innovative solutions are needed to overcome disease outbreaks [
216], which include empowering the continent to accelerate vaccine manufacturing. Speeding up and encouraging local production, vaccines can be supplied in a timely manner, contributing to removing some of the inequities in the provision of essential health products [
229]. In view of the creation of new expertise, this action will foster global collaborative research, which might also aid the discovery of therapeutic treatments better adjusted to the genetics of African individuals, while investigating the consequences of COVID-19 on diseases that are prevalent in the continent. Many pathologies were neglected during the pandemic and now are reemerging, such as measles in the Democratic Republic of Congo [
230]. The decreased number of awareness campaigns [
231] led to the false perception that the morbidity and mortality of certain pathologies were reduced, as was the case for HIV, cardiovascular diseases, and cervical cancers in Northern Ethiopia [
232]. Blood bank services and malaria testing also decreased in Rwanda by almost 200%. Conversely, neonatal death dramatically increased in South Africa, as most personnel were occupied in saving the population from COVID-19 [
233].
Finally, it is also worth mentioning that the impact of COVID-19 in Africa extended beyond public health, affecting social and economic aspects. Increased anxiety, social isolation, and stress characterized the pandemic period [
3], potentially contributing to enhance domestic violence. Tunisia (43%), Somalia (50%), and South Africa (69%) registered the highest increases [
234,
235]. A higher risk of child labor and exploitation was also detected [
236], possibly considered as a way for families to overcome the economic pressure imposed by the lockdown. These aspects contributed to a rise in the incidence of mental and psychosocial disorders [
3], enhancing the vulnerability of this continent.
This entry is adapted from the peer-reviewed paper 10.3390/covid4020014