Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The thionamide antithyroid agents were discovered largely through observations carried out by various researchers in the 1940s that found that sulfhydryl-containing substances were goitrogenic in animals. Prof. Edwin B. Astwood started using these drugs to treat hyperthyroidism. The development background of these agents, the coordination possibility of 2-thiouracil and its derivatives are presented herein.
- antibacterial activities
- cytotoxic properties
- uracil
- 6-methyl-2-thiouracil
- 6-propyl-2-thiouracil
- 2-thiouracil
1. Introduction
Uracil is a pyrimidine derivative identified in nucleic acids [1]. It is a pyrimidine base with four distinct binding sites, such as N1, N3, O2, and O4 atoms. It belongs to a group of pyrimidines that have a significant role in the structure and function of certain enzymes and medicines.
2-thiouracil has been found to inhibit thyroid hormone biosynthesis. In 1942, Prof. Edwin W. Astwood started using 2-thiouracil to treat patients with Graves’ disease [2]. The thyroid gland (Lat. Glandula thyr(e)oidea) is an important endocrine gland. It reproduces the hormones T3, T4, and calcitonin, whose main role is to stimulate the metabolism, growth, and development of cells and several organs. It should be noted that possible ways to treat patients with Graves’ disease include conservative treatment, which consists of taking antithyroid drugs—thiamazole (methizol, tyrozole) and propylthiouracil (propicillin), surgical treatment or radioactive iodine therapy. Methylthiouracil has been identified as an antithyroid drug. It enhances cell growth and proliferation, accelerates wound, ulcer, and burn healing, and increases resistance to infection. A characteristic feature of the drug is that it stimulates the effect of hematopoiesis (formation and strengthening of leukocytes and erythrocytes in bone marrow). It shows a similar mechanism of action and side effects as propylthiouracil. The drug acts to reduce the production of stored hormones, such as thyroglobulin in the thyroid gland.
Recently, Mao et al. have assessed the reduction effects associated with intrathyroidal injection of dexamethasone on the relapse rate of hyperthyroidism in individuals newly diagnosed with Graves’ disease [3]. At present, propylthiouracil is regarded as the preferred treatment for hyperthyroidism during pregnancy [4]. The literature contains various reviews on the subject [5][6][7][8].
Patil presented a review of the biological potency and the structure–activity relationship of pyrimidines such as antitumor, anti-HIV, anti-diabetic, antimicrobial, anti-inflammatory, analgesic, anthelmintic, CNS depressants, and cardiac agents [9]. Verbitskiy et al. reported a new antituberculosis drug among 1,3- and 1,4-diazines [10] (Scheme 1). Many new pyrimidine derivatives were both recently designed and developed for their antitumor properties. Mahapatra et al. focused on the structure–activity relationship (SAR) of pyrimidine derivatives as an antitumor drug in recent years [11]. Wu et al. synthesized new pyrimidine derivatives containing an amide moiety and assessed against different pathogenic fungi [12]. The new pyrimidine derivatives were obtained, characterized, and designed with regard to their anticancer activities [13].
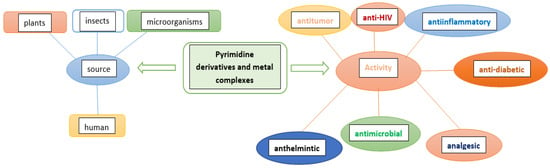
Scheme 1. Different applications of bioactive pyrimidines and their metal complexes.
Oladipo and Isola have published a thorough review of uracil’s coordination capabilities and the practical use of some of its complexes [14]. Recently, Masoud et al. have presented a review of complex properties and applications of some biological activities of nucleic acid [15]. In Figure 1 the chemical structure of uracil, 2-thiouracil, and some of their derivatives is given.
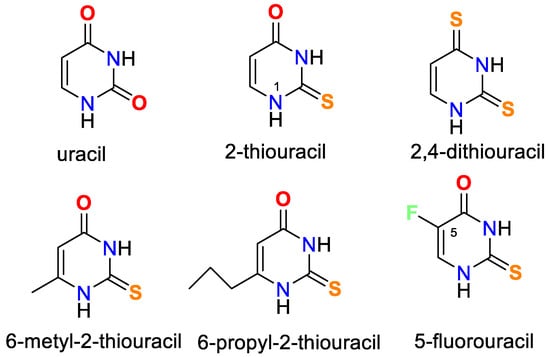
Figure 1. Structures of uracil, 2-thiouracil, and some of their derivatives.
It is worth noting that uracil, 2-thiouracil, 2,4-dithiouracil, and its derivatives are of chemical interest as they have four donor atoms for coordination with a metal ion/two O- and two N- or 1-O, 1-S- and 2-N atoms or 2 S- and 2-N atoms. It is evident that they demonstrate various possible ways to coordinate 2-thiouracil with metal ions, as follows in Figure 2 and Figure 3:
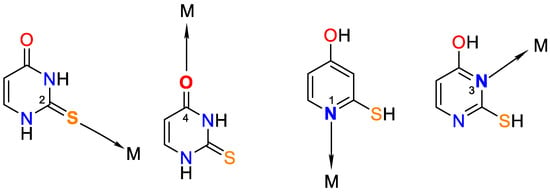
Figure 2. Monodentate coordination of 2-thiouracil with one of the four donor atoms, without any prior deprotonation (the tautomeric form participates in N1 and N3 coordination).
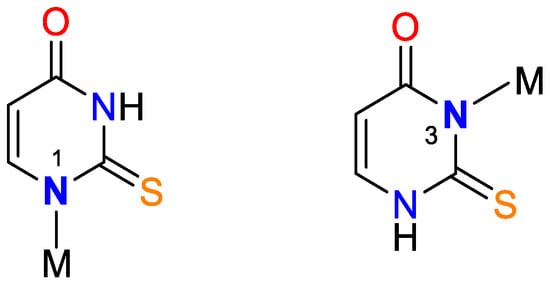
Figure 3. Monodentate coordination with deprotonated ligands that participate as a monoanionic (through a deprotonated nitrogen atom in the first position N1 or a nitrogen atom in the third position N3).
Different variants of chelating or bridge coordination are presented in Figure 4 and Figure 5.
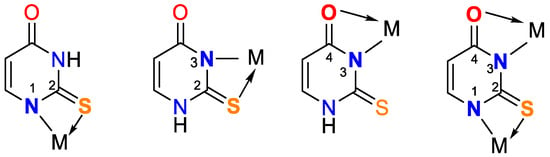
Figure 4. Bidentate coordination with formed chelate.
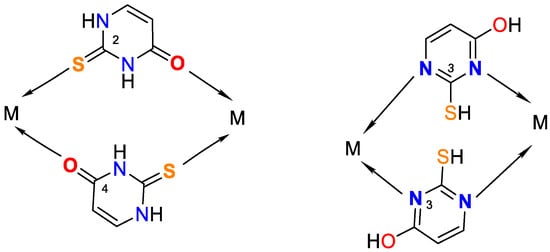
Figure 5. Possible bridge ways of 2-thiouracil coordination with metal ions.
It presents the biological activity of 2-thiouracil and metal complexes: some of them are used as agents for fighting tuberculosis and arthritis, others have bactericidal and fungicidal action, and the third is cytotoxic activity.
2. Synthesis of Metal Complexes with Uracil and Its Derivatives
Narang et al. [16] have obtained complexes of Fe(III) and Cr(III) with uracil through the basic formula [M(Uracil)(H2O)2–(OH)Cl], where M = Cr(III) or Fe(III). The complexes have been characterized via elemental analysis, UV-Vis, EPR, and IR spectroscopic methods. Based on the obtained results, the authors suggest a polymer structure with an octahedral geometry of the metal center, with the ligand coordinated through the O(4)- and N(1)-atoms, while histidine coordinates through the O atom of –CO2− and the N atom of the –NH2 groups. Cartwright et al. [17] have obtained a bis-(1,3-dimethyluracil)-dichloridocopper(II) complex, which has been studied via elemental analysis, IR spectroscopy, and X-ray diffraction. The data from the analysis show that the uracil coordinates to the metal center monodentate with the participation of an O-atom in the fourth position of the pyrimidine ring and two chloride ions coordinate to Cu(II), so the complex is neutral with a planar-square geometry. The complexation structure is given in Figure 6.
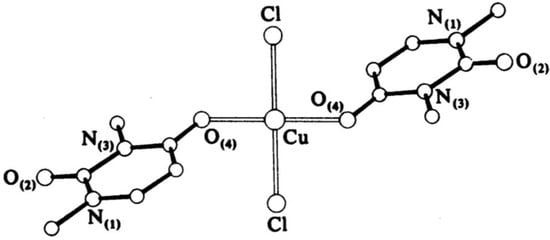
Figure 6. The structure of bis-(1,3-dimethyluracil)-dichloridocopper(II) complex [17].
A complex of Fe(III) with uracil and complexes of thiouracil and 5-(phenylazo)-thiouracil with Co(II), Ni(II), and Cu(II) have been obtained [18]. The new metal complexes have been characterized via elemental analysis, DTA, UV-Vis, IR, and Mössbauer spectroscopy. In all complexes, uracil and other free ligands act in a bidentate fashion with coordination occurring through the O(4)- and N(3)-atoms. The coordination bond lengths of the octahedral Co(II) and Ni(II) complexes are smaller compared to the square-planar Cu(II) complex [18]. In Figure 7 the suggested structure of iron(III)-uracil complex is given.
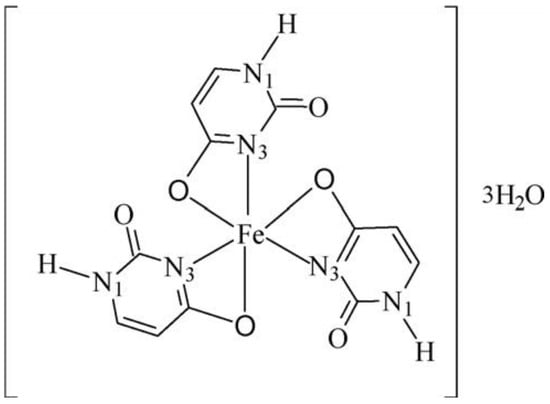
Figure 7. Suggested structure of the iron(III)–uracil complex [18].
Ghosh et al. [19] have synthesized new complexes of uracil with a general formula: [ML2(H2O)2], where M = Mn, Fe, Co, Ni, or Cu; L = uracil. Electronic spectra indicate octahedral coordination for all complexes and data from comparative IR spectra show chelate structure via the O(2)- and N(3)-atoms of uracil. In Figure 8 the proposed structure of the complexes is given.
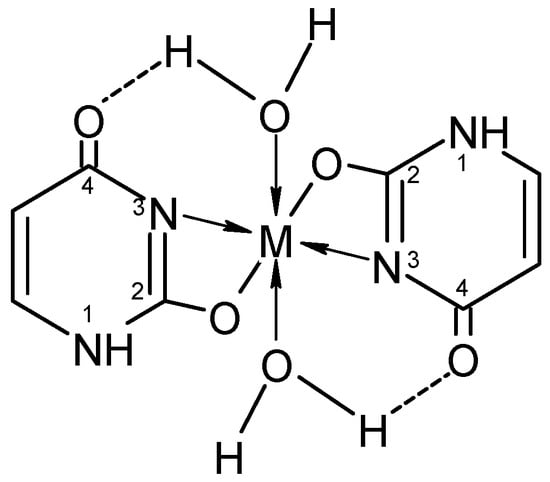
Figure 8. The proposed structure of the complexes [19].
The synthesis, spectroscopic analysis, and investigation of the biological properties of Ni(II), Cu(II), and Co(II) complexes formed by Schiff base ligands obtained from 5-aminouracil, 2-hydroxy-1-naphthaldehyde, 2,4-dihydroxybenzaldehyde, and salic-ylaldehyde have been reported [20]. In each instance, the complexes seem to exist as monomers. The ligands exhibit bidentate coordination with Ni(II) and Co(II), while with Cu(II), they coordinate in a tridentate manner, involving the carbonyl oxygen atom in the fourth position of the uracil ring. In Figure 9 the proposed structure of new metal complexes is presented.
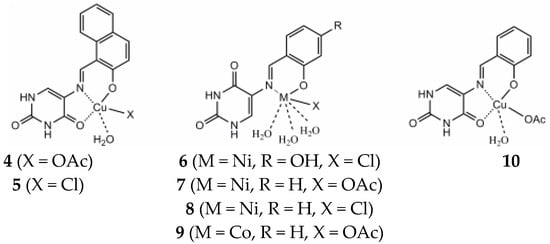
Figure 9. Proposed structures of the metal complexes [20].
The complexes of M2+, M = Co, Ni, and Zn with 6-chloromethyluracil, 5-hydroxymethyluracil, uracil, 6-methyluracil, and dimethyl-6-uracilmethylphosphonate have been synthesized [21]. Based on the data obtained, we may assume that the ligands are coordinated to the metal ion through the N(3)-atom of Co(II) and Ni(II), as well as a hydroxo complex MLH−1 with deprotonated water of the inner coordination sphere [21]. Mixed ligand complexes play a crucial role in different biological environments. Some mixed ligand complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) have been obtained by Tyagi et al. by using the corresponding nitrates as starting metal salts and 5-fluorouracil and histamine [22]. New complexes have been characterized by elemental analysis, UV-Vis, and IR spectroscopy, as well as by the X-ray diffraction method. Data from X-ray structural analysis indicates that 5-fluorouracil is coordinated through the N(3)-atom. Mixed ligand complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) with adenine and uracil have been obtained [23]. The complexes have been characterized via elemental analysis, UV-Vis, and IR spectroscopy, as well as using powder X-ray diffraction studies. The authors suggest the complexes may be polymeric in nature using adenine, as well as –OH group as bridging ligands. During polymerization, one metal atom is bound via the N(3)-atom of one adenine ligand and the N(7)-atom of another adenine ligand. Uracil acted in coordination via O-atom in the second position of the pyrimidine ring [23]. Based on the results obtained, Abdullah suggests a polymer structure with an octahedral geometry of the metal center for all synthesized complexes. Mixed ligand complexes of Th(II), Ce(II), and Gd(II) with uracil and omeprazole have been synthesized as per the general formula: [M(Ome)(ura)·4H2O)]SO4·xH2O [24]. The compounds have been researched via elemental analysis, UV-Vis, IR-, Mass, and 1H NMR spectroscopy. Based on the data obtained, it is suggested that uracil acts as a bidentate ligand, binding through the N(3)- and O(2)-atoms [24]. In Figure 10 the suggested structure of metal complex is given.
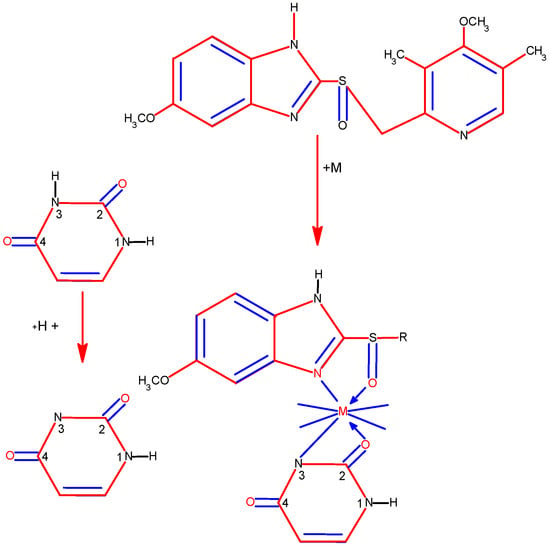
Figure 10. Proposed scheme of formation of representative ternary complex (M-OME-URA) [24].
Mixed ligand complexes of Ni(II), Cu(II), and Zn(II) with 5-fluorouracil and amino acids have been obtained by Shobana et al. [25]. Magnetic moment values together with the electronic spectral data indicate 5-fluorouracil coordinates with the metal ion in a bidentate manner through the C(4)=O and N(3) atoms. They also prescribe the amino acids behavior as bidentate by nitrogen and carboxylate oxygen with formed 4, 5, and 6-membered chelate rings. All the Cu(II) mixed ligand compounds demonstrate a distorted tetrahedral geometry, further supported by the ESR studies. In Figure 11 the suggested structure of metal complexes is presented.
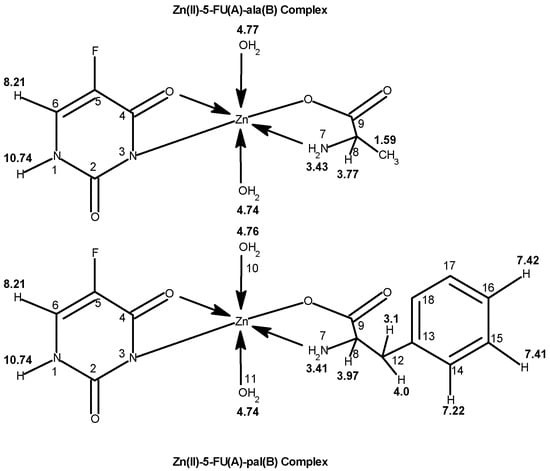
Figure 11. The structure of metal complexes [25].
Cu(II), Ni(II), Co(II), and Zn(II) mixed ligand complexes, comprising alanine in conjunction with either uracil or 2-thiouracil, have been synthesized and characterized [26]. The findings indicate alanine consistently exhibits bidentate coordination, utilizing both –NH2 and COO− groups. Uracil acts as a bidentate ligand in the Cu(II) complex, coordinating via one carbonyl O- and N-atoms, while in other instances, it is coordinating solely via N-atom, as monodentate. In the presence of thiouracil, Cu(II) and Ni(II) complexes involve coordination from carbonyl O- and one N-atoms, whereas the Co(II) complex coordination occurs from S- and N-atoms. The Zn(II) complex displays tridentate behavior, coordinating through O-, S-, and N-atoms. The mixed complexes of Cu(II), Co(II), and Zn(II) with uracil, as well as Ni(II) and Zn(II) complexes with thiouracil, exhibit octahedral geometry. The mixed Ni(II) complex with uracil displays distorted tetrahedral geometry, while the mixed Co(II) thiouracil complex adopts a square-planar structure. Notably, the mixed Cu(II) thiouracil complex features a binuclear structure with a square-planar arrangement around each copper atom [26]. The suggested structure of the complexes is given in Figure 12, Figure 13, Figure 14 and Figure 15.
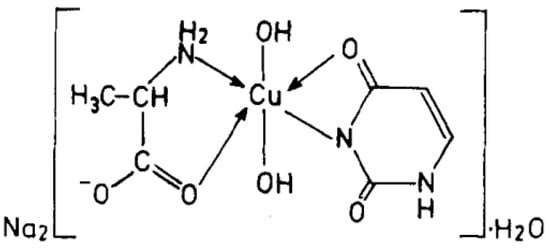
Figure 12. Cu(II)-Alanine-Uracil Complex [26].
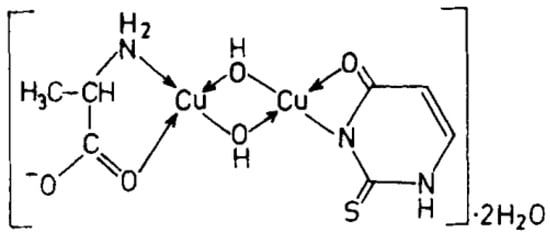
Figure 13. Cu(II)-Alanine-2-Thiouracil Complex [26].
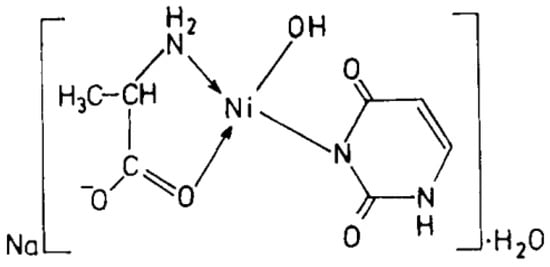
Figure 14. Ni(II)-Alanine-Uracil Complex [26].
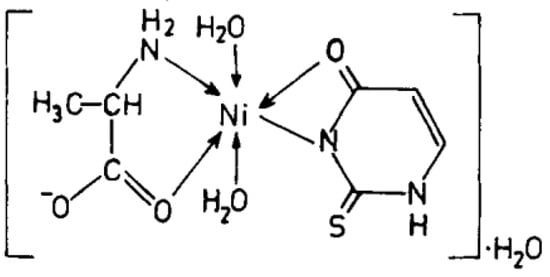
Figure 15. Ni(II)-Alanine-2-Thiouracil Complex [26].
Srivastava et al. have published papers on the synthesis and characterization of mixed ligand complexes of glycine and uracil or 2-thiouracil, thymine or adenine with histidine and uracil, thymine or 2-thiouracil with glycine, alanine, valine, and leucine with Cu(II), Ni(II), Co(II), and Zn(II) [27][28][29][30].
3. Synthesis of Metal Complexes with 2-Thiouracil and Its Derivatives
Recently, researchers have reported on Cu((II), Pd(II), and Au(III) complexes with 2-thiouracil [31]. The compound structure has been analyzed with UV-Vis, IR, 1H, 13C NMR, and Raman spectral data. The interpretation of complex spectra is assisted by the data for 2-thiouracil obtained from 1H–1H COSY, DEPT-135, HMBC, and HMQC spectra. The spectral data reveal the bonding of the ligand through sulfur, oxygen, and nitrogen atoms in Cu(II) and Pd(II) complexes and through S and N atoms in the complex of gold. In addition, the antimicrobial activity against both Gram-positive and Gram-negative bacteria and yeasts has also been studied. New complexes of Cu(II) and Pd(II) with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil have been obtained [32]. All metal complexes have been obtained after mixing water solutions of the corresponding metal salts and the ligand dissolved in DMSO and water solutions of NaOH, in a metal-to-ligand ratio of 1:4:2. The compound structure has been reviewed based on melting point analysis, MP-AES for Cu and Pd, UV-Vis, IR, ATR, 1H NMR, 13C NMR, and Raman spectroscopy. The interpretation of complex spectra is assisted by the data for 6-methyl-2-thiouracil and 6-propyl-2-thiouracil obtained from 1H-1H COSY, DEPT-135, HMBC, and HMQC spectra [32]. The researchers have suggested that in the Cu(II)L1 complex, the ligand could probably coordinate in a monodentate way through S2 and/or O4 atoms. The coordination binding site we would suggest for 6-propyl-2-thiouracil in Cu(II)L2 is a monodentate coordination mode binding through S-atom. For Pd(II)L1 and Pd(II)L2 complexes, researchers suggested that one of the ligands participates in coordination via N1 and S2 atoms and others with N3 and O4 atoms. In all the complexes, the solvent DMSO acts as a ligand (one molecule coordinate in Cu(II)L1 and Pd(II)L1 and two molecules in Cu(II)L2 and Pd(II)L2). The formation of polymeric complexes in a solid state has also been suggested, whereas dissolution in DMSO decreases. The suggested structure of the new complexes is given in Figure 16.
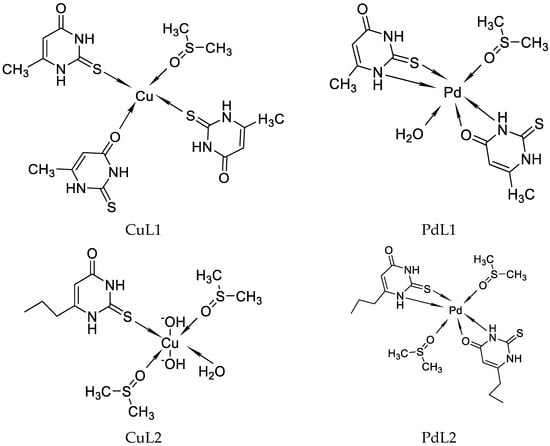
Figure 16. The representation of suggested coordination binding sites for 6-metyl-2-thiouracil and 6-propyl-2-thiouracil [32].
Complexes of Cu(I), Ni(II), Co(II), Zn(II), Ag(I), Cd(II), and Hg(II) with 6-amino-2-thiouracil have been synthesized, isolated, and studied by thermogravimetric analysis, differential scanning calorimetry (DSC) and IR spectroscopy [33]. Based on the results obtained, the authors suggest that the ligand acts as a monoanion and coordinates via the N-atom in the complexes of Cu(I), Ni(II), Co(II), Zn(II), Ag(I), and Cd(II), and via the S-atom in Hg(II) and Ni(II). The Zn(II) and Cd(II) complexes are assumed to be polymeric, and the ligand is bridged and acts as a dianion. It is crucial to mention the water in the first six complexes is both coordination and crystallization, whereas in the last it is only crystallization [33]. Complexes of 6-amino-2-thiouracil with Ni(II), Co(II), Zn(II), Cd(II), Cu(I), Ag(I), and Hg(II) have been obtained by Romero et al. [34]. The complexes have been studied via elemental analysis, IR-, UV-Vis, and NMR spectroscopy, as well as magnetochemical measurements. The structure of one of these has been established by X-ray structure analysis [Zn(6-amino-2-thiouracil)2H2O]·2H2O, in which the ligand is coordinated bidentate chelate through S-atom in second and N-atom in the first position. The structure of the new Zn(II) complex is given in Figure 17.
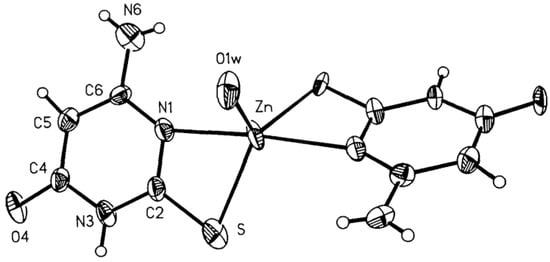
Figure 17. View of the molecule of [Zn(6-amino-2-thiouracil)2H2O]·2H2O 50% probability thermal ellipsoids are shown [34].
It is important to note that complexes of 2-thiouracil with Cu(II), Ni(II), Co(II), and Fe(III) have been synthesized [35]. The compounds have been analyzed via IR and UV-Vis spectroscopic methods, as well as magnetochemical measurements and DTA (differential thermal analysis). Based on the results obtained, the authors suggest that 2-thiouracil has coordinated to the metal center via a deprotonated N-atom and via an S-atom from the thiocarbonyl group in the Cu(II) and Ni(II) complexes; in addition, there is the presence of two water molecules. In Figure 18 the proposed structure of Ni(II) complexes is given. The authors also suggest, that in the complex of Co(II), one ligand is not deprotonated and binds via an S-atom from the thiocarbonyl group and an O-atom from the carbonyl group. For the Fe(III) complex, coordination is most likely via N- and O-atoms [35]. All of these complexes exhibit high insolubility in typical organic solvents, indicating their likely polymeric nature. As a result, the structure of the nickel(II) complex seems to be octahedral, while the structure of the other complexes remains uncertain. However, tentative structures are proposed as follows:
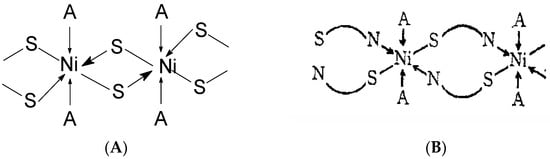
Figure 18. Polymeric structure with thio bridges as in structure (A); polymeric as shown in structure (B) (A = H2O or py) [35].
-
Fe(III) complex—octahedral;
-
Co(II) complex—A tetrahedral structure has been observed, where each cobalt atom within the dimer forms bonds with three nitrogen atoms and one water molecule. Four ligands exhibit monodentate bonding in the dimer, connecting through deprotonated nitrogen. The fifth ligand has both nitrogen atoms protonated, with each nitrogen atom bonding to one cobalt atom [35];
-
Cu(II) complex—octahedral possibly having Cu–Cu bond [35].
Garrett et al. have reported on complexes of 2-thiouracil, 6-n-propyl-2-thiouracil, 6-methyl-2-thiouracil, 5-methyl-2-thiouracil, 5,6-dimethyl-2-thiouracil, 2-ethylmercapto-4-hydroxypyrimidine, and 6-methyl-N,N′-diethyl-2-thiouracil with Cu(II), Cd(II), Pb(II), Fe(II), and Fe(III) [36]. The structure of some of metal complexes synthesized by Garrett et al. is given in Figure 19.
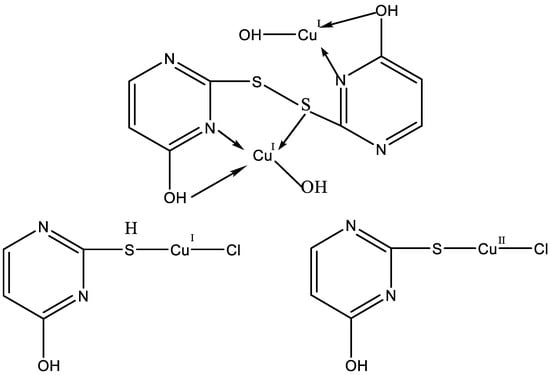
Figure 19. The structure of some of metal complexes synthesized by Garrett et al. [36].
Dozens of complexes have been prepared and isolated using chlorides, bromides, iodides, sulfates, and nitrates of Co(II), Zn(II), and Ni(II) with 5-morpholinomethyl-2-thiouracil [37]. The complexes were formed by mixing a solution of the ligand in 2-propanol and the metal salt in ethanol, the molar ratio was 2:1, and the temperature of the reaction mixture was 70 °C. The suggested structure of the new complexes is given in Figure 20.
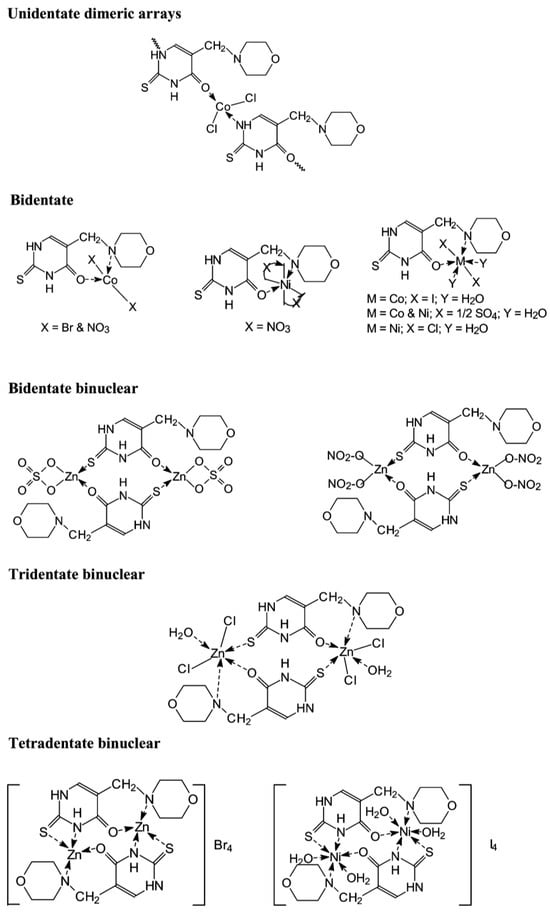
Figure 20. The structure of the metal complexes reported by Kamalakannan et al. [37].
Complexes of 2-thiouracil and 6-methyl-2-thiouracil and their derivatives with W(CO)5 have been obtained [38]. One of the compound structures has been established via X-ray structural analysis. The authors prove the ligand coordinated with the metal ion through the S-atom of the 2-thiouracil. The pentacarbonyl complexes of 2-thiouracilate and 6-methyl-2-thiouracilate have been demonstrated to undergo cis CO dissociation with stereoselectivity, leading to the concurrent formation of tetracarbonyl derivatives chelated by the exocyclic sulfur and the endocyclic N(1) [38]. A mononuclear complex with the general formula [CuL(NH3)4]Cl2·0.5H2O and three heterometallic complexes [Cu2Ni(L)2(NH3)2Cl2.6H2O]·2H2O, [Cu3Co(L)4·8H2O]Cl·4.5H2O, and [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]·4H2O, where the ligand is 2-thiouracil has been obtained [39]. The complexes have been analyzed via elemental analysis, magnetochemical studies, and IR, as well as UV-Vis, EPR, TG, DTG, and DTA. The results show the ligand acts bidentate or tetradentate, with the geometry of the metal center on an octahedron, except for [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]·4H2O, in which a planar-square structure of Co(II), Ni(II), and Cu(II) is suggested. In Figure 21 the proposed structure of mononuclear and hetero-metallic complexes is given.
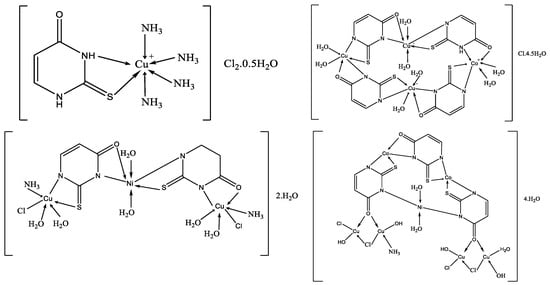
Figure 21. Proposed structures for mononuclear copper(II) complex and its hetero-metallic complexes [39].
Papazoglou et al. have prepared a dinuclear complex of Cu(I) (CuX, where (X = Cl, Br, I)) involving 5-carbethoxy-2-thiouracil (eitotH2) as a ligand with the general formula [CuX(eitotH2)2]2 [40]. The molecular crystal structure of the complex is presented in Figure 22 and mixed-ligand mononuclear complexes of Cu(I) with the general formula [CuX(PPh3)2(eitotH2)], the crystal structure of which is presented in Figure 23.
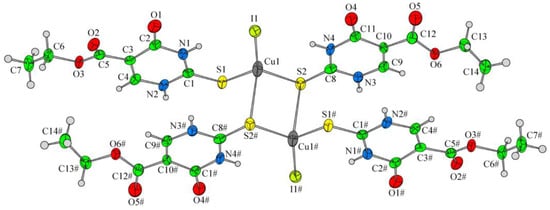
Figure 22. Molecular crystal structure of Cu(I) dinuclear complex reported by Papazoglou et al. [40].
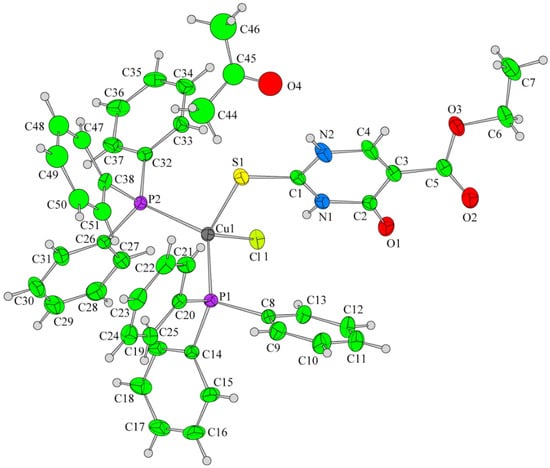
Figure 23. Molecular crystal structure of mononuclear Cu(I) complex with general formula [CuX(PPh3)2(eitotH2)] reported by Papazoglou et al. [40].
New dinuclear copper(I) complexes with 5-carbethoxy-2-thiouracil have been synthesized recently [41].
It is known that heavy metal compounds such as platinum, gold, rhodium, palladium, ruthenium, and others can be used as biologically active agents in chemotherapy and for the treatment of various types of human tumors. We should take into consideration the coordination compounds of Pd with amino acids, catecholamines, and some heterocyclic nitrogen-containing compounds that have found application as immunomodulators, facilitating the recovery of cells after radiation damage [42]. The treatment with gold preparations, called chrysotherapy, was known as early as 2500 BC in China. In the form of official pharmaceuticals, Au compounds were used in the 1920s [42].
New complexes of rhenium(I) involving certain 5-nitrosopyrimidines, characterized with the general formula [ReCl(CO)3L], have been synthesized and identified through elemental analysis, conductivity measurements, and spectroscopic methods including IR, 1H, 13C, and 15N NMR. [43]. The complexes seem to exist as monomers, where the pyrimidine ligands function in a neutral form. The structure of [ReCl(CO)3(DANU)]·CH3CN has been elucidated through X-ray diffraction. The coordination environment around Re(I) is best described as a distorted octahedron, with the ligand adopting a bidentate configuration through N5 and O4 atoms, forming a five-membered chelate ring.
Abou-Melha has synthesized new complexes of VO(II), Ni(II), Pd(II), Pt(IV), and UO2(II) with N-(4-((Z)-(6-oxo-2-thioxo-1,2,3,4-tetrahydro-6l5-pyrimidin-5-yl)diazenyl)phenyl)-4-((E)-(6-oxo-2-thioxo-1,2,5,6-tetrahydropyrimidin-5-yl)diazenyl)benzamide [44]. The structure of the complexes has been studied by IR, UV-Vis, 1H NMR, EPR, 13C NMR, TGA, TEM, and XRD methods. The structure of new metal complexes is given in Figure 24.
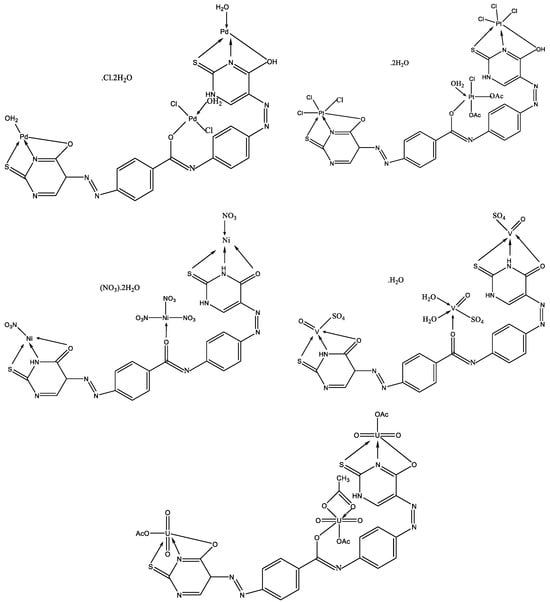
Figure 24. The geometries of all investigated complexes [44].
The interest in platinum and palladium complexes originates from their high cytostatic activity. Recently, cis-dihalogeno complexes of platinum(II) and palladium(II) with 6-tert-butyl-2-thiouracil have been synthesized [45]. The structure of platinum and palladium complexes with 6-tert-butyl-2-thiouracil is presented in Figure 25.
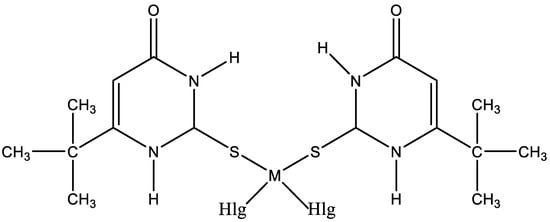
Figure 25. The structure of platinum and palladium complexes with 6-tert-butyl-2-thiouracil [45].
To date, numerous metal complexes of uracil and thiouracil derivatives have been synthesized and their composition and structure with various metals like copper, iron, cobalt, nickel, zinc, manganese, cadmium, and vanadium [18][26][46][47][48], as well as palladium, platinum, and gold have been studied [49]. The suggested structure of metal complexes with 2-thiouracil proposed by Masoud et al. is given in Figure 26. In Figure 27 the structure of some complexes is presented.
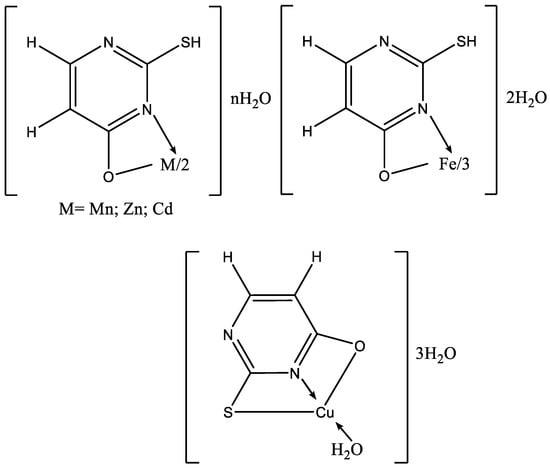
Figure 26. The suggested structure of metal complexes with 2-thiouracil proposed by Masoud et al. [47].
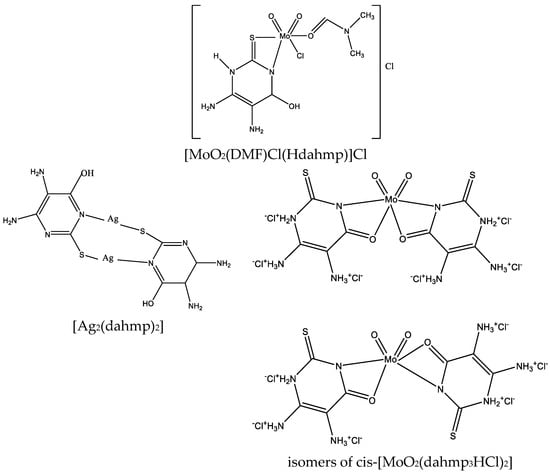
Figure 27. Structure of [MoO2(DMF)Cl(Hdahmp)]Cl, [Ag2(dahmp)2], and isomers of cis-[MoO2(dahmp3HCl)2] [49].
New thiolate gold(I) complexes with P(NMe2)3 (HMPT) as a phosphane group have been synthesized [Au(SR)(HMPT)] (R = Spy, Spyrim, SMe2pyrim, Sbenzothiazole, Sthiazoline, Sbenzimidazole and 2-thiouracil). Two of the thiolate gold(I) complexes appear suitable effective candidates to be used in chemotherapy [50]. The chemistry of AuIII is much less developed than that of the isoelectronic and often isostructural PtII atom. The first crystallographically characterized AuIII complex with a pyrimidine derivative is the N(3)-bonded compound with 1-methylcytosine [51]. In the same manner, a gold complex has been isolated [52] by reacting HAuCl4 with 6-amino-1,3-dimethyl-5-(2-chlorophenylazo)uracil (DZCH) to give [Au(DZC)Cl2] complex. X-ray diffraction has demonstrated that the crystal of [Au(DZC)Cl2] contains individual complex molecules where the metal has a slightly distorted square-planar coordination. Two cis corners are occupied by Cl ligands. The uracil derivative has formed a six-membered chelate ring via the deprotonated amino group and the phenylsubstituted nitrogen atom of the azo group [52]. Metal complexes with 5-carboxy-2-thiouracil were synthesized by Singh et al. [53]. The structure of complexes with 5-carboxy-2-thiouracil is given in Figure 28.
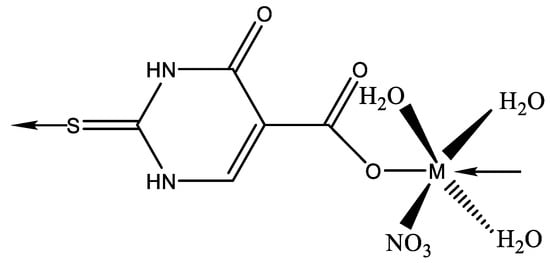
Figure 28. The structure of complexes with 5-carboxy-2-thiouracil M(II) = Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) [53].
Metal complexes of Ni, Cu, and Mn with mixed ligands involving 2-thiouracil and 8-hydroxyquinoline (1–3), as well as with 2-hydroxyquinoline (4–6), were synthesized [54]. An assessment of their antimicrobial and antioxidant properties was conducted. The structure of Cu, Ni, Mn complexes is given in Figure 29.
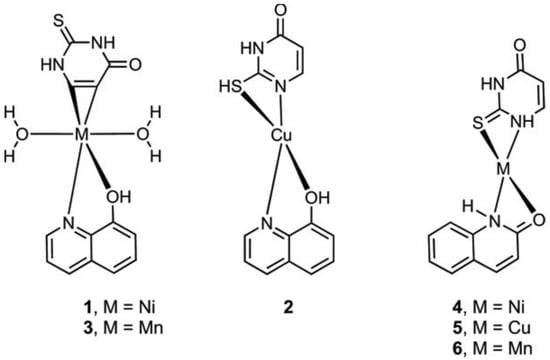
Figure 29. The structure of Cu, Ni, and Mn complexes [54].
-
Summary data on the structure of the complexes and the donor atoms involved in the coordination is given in Table 1.
Table 1. Summary data on the structure of the complexes and the donor atoms involved in the coordination.
| Technique | Donor Atom | Metal | Structure | References |
|---|---|---|---|---|
| X-ray | O | Cu(II) | square planar | [17] |
| X-ray | N1 and S2 | Zn(II) | trigonal bipyramid | [34] |
| X-ray | S; N1 and S2 |
W(0); W(0) |
octahedron; chelate |
[38] |
| X-ray | S for dimer; S, P, and Cl; S, P, and Br |
Cu(I) | pseudotetrahedral environment | [40] |
| X-ray | N5 and O4 | Re(I) | distorted octahedron | [43] |
| X-ray | S and P | Au(I) | linear | [50] |
| X-ray | N3 | Au(III) | square-planar coordination geometry |
[51] |
| X-ray | N6 and N8 | Au(III) | distorted square planar | [52] |
| elemental analysis, DTA, UV-Vis, IR, and Mössbauer spectroscopy | O4 and N3 | Fe(III), Co(II) and Ni(II); Cu(II) | octahedral; square planar |
[18] |
| UV-Vis and IR spectroscopy | O2 and N3 | Mn(II), Fe(II), Co(II), Ni(II), Cu(II) | octahedral (chelate) | [19] |
| elemental analysis, UV-Vis, and IR spectroscopy | O and N O, N, and O |
Ni(II), Co(II); Cu(II) |
octahedral (chelate); trigonal bipyramid |
[20] |
| Potentiometric Studies, UV-Vis, ion-selective electrode titrations | N3 | Ni(II), Co(II); Zn(II) |
[21] | |
| elemental analysis, UV-Vis, IR spectroscopy, powder X-ray diffraction studies | N3 | Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) | polymer structure with an octahedral geometry | [23] |
| elemental analysis, UV-Vis, IR-, Mass- and 1H NMR spectroscopy | N3 and O2 | Th(II), Ce(II), Gd(II) | [24] | |
| elemental analysis, spectral (vibrational, electronic, 1H NMR, ESR) |
N3 and O4 | Ni(II), Cu(II), Zn(II) | distorted tetrahedral geometry for Cu(II); octahedral for Ni(II); distorted octahedral for Zn(II) | [25] |
| elemental analyses, IR, UV-Vis, magnetochemical measurements |
O and N (uracil); O, N, and S (2-thiouracil) |
Cu(II), Ni(II), Co(II), Zn(II) | square planar for Cu(II) and Co(II) with 2-thiouracil; Cu(II), Co(II), and Zn(II) with uracil, Ni(II), and Zn(II) complexes with thiouracil–octahedral geometry, Ni(II) with uracil-distorted tetrahedral | [26] |
| thermogravimetric analysis, differential scanning calorimetry (DSC), IR spectroscopy | N S |
Cu(I), Ni(II), Co(II), Zn(II), Ag(I), Cd(II); Hg(II) and Ni(II) |
Zn(II) and Cd(II)–polymeric structure | [33] |
| IR, UV-Vis differential thermal analysis | N and S O and S N and O |
Cu(II), Ni(II), Co(II), Fe(III) |
octahedral tetrahedral octahedral |
[35] |
| elemental analysis, magnetochemical studies, and IR, UV-Vis, EPR, TG, DTG, and DTA | N and S for mononuclear N1, O, S, N3 for hetero-metallic |
Cu(II), Ni(II), Co(II) | All octahedron, except one planar-square structure of Co(II), Ni(II) and Cu(II) | [39] |
| IR and NMR spectroscopy | S | Pd(II), Pt(II) | square planar or tetrahedral | [45] |
| elemental analysis, IR, UV-Vis, powder X-ray diffraction | O and S | Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) | octahedral | [53] |
This entry is adapted from the peer-reviewed paper 10.3390/compounds4010010
References
- Garrett, R.H.; Grisham, C.M. Principles of Biochemistry with a Human Focus; Brooks/Cole Thomson Learning: Pacific Grove, CA, USA, 2001; p. 939. ISBN 0-03-097369-4.
- Astwood, E.B. The chemical nature of compounds which inhibit the function of the thyroid gland. J. Pharmacol. Exp. Ther. 1943, 78, 79–89.
- Mao, X.-M.; Li, H.-Q.; Li, Q.; Li, D.-M.; Xie, X.-J.; Yin, G.-P.; Zhang, P.; Xu, X.-H.; Wu, J.-D.; Chen, S.-W.; et al. Prevention of Relapse of Graves’ Disease by Treatment with an Intrathyroid Injection of Dexamethasone. J. Clin. Endocrinol. Metab. 2009, 94, 4984–4991.
- Rosenfeld, H.; Ornoy, A.; Shechtman, S.; Diav-Citrin, O. Pregnancy outcome, thyroid dysfunction, and fetal goiter after in utero exposure to propylthiouracil: A controlled cohort study. Br. J. Clin. Pharmacol. 2009, 68, 609–617.
- Cooper, D.S. Antithyroid Drugs. N. Eng. J. Med. 2005, 352, 905–917.
- Volpé, R. The Immunomodulatory Effects of Anti-thyroid Drugs are Mediated via Actions on Thyroid Cells, Affecting Thyrocyte-immunocyte Signalling: A Review. Curr. Pharm. Des. 2001, 7, 451–460.
- Burch, H.B.; Cooper, D.S. Antithyroid drug therapy: 70 years later. Eur. J. Endocrinol. 2018, 179, R261–R274.
- Fernandez, M.G. Hyperthyroidism and pregnancy. Endocrinol. Nutr. 2013, 60, 535–543.
- Patil, S.B. Recent medicinal approaches of novel pyrimidine analogs: A review. Heliyon 2023, 9, e16773.
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N. Development of new antituberculosis drugs among of 1,3- and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. Int. Ed. 2019, 68, 2172–2189.
- Mahapatra, A.; Prasad, T.; Sharma, T. Pyrimidine: A review on anticancer activity with key emphasis on SAR. Futur. J. Pharm. Sci. 2021, 7, 123.
- Wu, W.; Lan, W.; Wu, C.; Fei, Q. Synthesis and Antifungal Activity of Pyrimidine Derivatives Containing an Amide Moiety. Front. Chem. 2021, 9, 695628.
- Tyli´nska, B.; Wiatrak, B.; Czyznikowska, Z.; Cie´sla-Niechwiadowicz, A.; Gebarowska, E.; Janicka-Kłos, A. Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 3825.
- Oladipo, M.A.; Isola, K.T. Coordination Possibility of Uracil and Applications of Some of Its Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 386–394.
- Masoud, M.S.; Ramadana, M.S.; Ramadana, A.M.; Al-Saify, M.H. Complexing Properties and Applications of Some Biologically Active Nucleic Acid Constituents. Int. J. Innov. Res. Technol. Sci. Eng. 2020, 6, 23–39.
- Narang, K.K.; Singh, V.P.; Bhattacharya, D. Synthesis, characterization and antitumor activity of uracil and uracil–histidine complexes with metal(III) ions. Trans. Metal. Chem. 1997, 22, 333–337.
- Cartwright, B.A.; Goodgame, M.; Johns, K.W.; Skapski, A.C. Strong Metal-Oxygen Interaction in Uracils. X-ray crystal structure of bis-(1,3-dimethyluracil)dichlorocopper(II). Biochem. J. 1978, 175, 337–339.
- Masoud, M.S.; Ibrahim, A.A.; Khalil, E.A.; El-Marghany, A. Spectral properties of some metal complexes derived from uracil–thiouracil and citrazinic acid compounds. Spectrochim. Acta Part A 2007, 67, 662–668.
- Ghosh, P.; Mukhopadhyay, T.K.; Sarkar, A.R. Interaction of Divalent Metal Ions with Uracil III. Complexes of MnII, FeII, CoII, NiII and Cull with Uracil Acting as Bidentate Ligand. Trans. Metal Chem. 1984, 9, 46–48.
- Koz, G.; Kaya, H.; Astley, D.; Yaşa, İ.; Astley, S.T. Synthesis, Characterization and Antimicrobial Screening of Ni(II), Cu(II) and Co(II) Complexes of Some Schiff Base Ligands Derived from 5-Aminouracil. Gazi Univ. J. Sci. 2011, 24, 407–413.
- Kufelnicki, A.; Jaszczak, J.; Kalinowska-Lis, U.; Wardak, C.; Ochocki, J. Complexes of Uracil (2,4-Dihydroxypyrimidine) Derivatives Part III. pH-Metric, ISE, and Spectrophotometric Studies on Co(II), Ni(II), and Zn(II) Complexes. J. Solution Chem. 2006, 35, 739–751.
- Tyagi, S.; Singh, S.M.; Gencaslan, S.; Sheldrick, W.S.; Singh, U.P. Metal-5-fluorouracil-histamine complexes: Solution, structural, and antitumor studies. Metal Based Drugs 2002, 8, 337–345.
- Abdullah, A.A. Synthesis, structural studies of some nucleic acids metal complexes. Basrah J. Sci. 2006, 24, 115–128.
- Verma, S.; Shrivastva, S.; Rani, P. Synthesis and spectroscopic studies of mixed ligand complexes of transition and inner transition metals with a substituted benzimidazole derivative and RNA bases. J. Chem. Pharm. Res. 2012, 4, 693–699.
- Shobana, S.; Dharmaraja, J.; Kamatchi, P.; Selvaraj, S. Mixed ligand complexes of Cu (II)/Ni (II)/Zn (II) ions with 5-Fluorouracil (5-FU) in the presence of some amino acid moieties: Structural and antimicrobial studies. J. Chem. Pharm. Res. 2012, 4, 4995–5004.
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of complexes of copper(II), nickel(II), cobalt(II) and zinc(II) with alanine and uracil or 2-thiouracil. Synth. React. Inorg. Met.-Org. Chem. 1996, 26, 305–320.
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of mixed ligand complexes of copper(II), nickel(II), cobalt(II and zinc(II) with glycine and uracil or 2-thiouracil. Polyhedron 1985, 4, 475–479.
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of complexes of copper (II), nickel (II), cobalt (II) and zinc (II) with histidine and uracil, thymine or 2-thiouracil. Bull Chem. Soc. Fr. 1991, 128, 859.
- Gupta, M.; Srivastava, M.N. Synthesis and Characterization of Mixed-Ligand Complexes of Copper (II), Nickel (II), Cobalt (II) and Zinc (II) With Glycine and Thymine or Adenine. Bull. Pol. Acad. Sci. 1992, 40, 277–285.
- Saxena, V.K.; Srivastava, M.N. PMR Spectral Studies of ktixed-Ligand Amino Acid Chelates of Cobalt(II), Nickel(II), Copper(II) and Zinc(II) with Nitrilotriacetic Acid and glycine, α-alanine, Valine, or Leucine. J. Inorg. Bio-Chem. 1990, 38, 37.
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies. Russ. J. Gen. Chem. 2022, 92, 1578–1584.
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization and Antibacterial Studies of new Cu(II) and Pd(II) complexes with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil. Appl. Sci. 2023, 13, 13150.
- Moreno-Carretero, M.N.; Romero-Molina, M.A.; Salas-Peregrin, J.M.; Sanchez-Sanchez, M.P. Thermal analysis applied to the study of metal complexes: Thermal behaviour of 6-amino-2-thiouracil and its complexes with several transition metal ions. Thermochim. Acta 1992, 200, 271–280.
- Romero, M.A.; Sanchez, M.P.; Quiros, M.; Sanchez, F.; Salas, J.M.; Moreno, M.; Faure, R. Transition metal complexes of 6-amino 2-thiouracil; crystal structure of bis(6-amino-2-thiouracilato)aquazinc(II) dihydrate. Can. J. Chem. 1993, 71, 29–33.
- Khullar, I.P.; Agarwala, U. 2-Mercaptopyrimidin-4-ol (2-Thiouracil) Complexes of Copper(II), Nickel(II), Cobalt(II) and Iron(III). Aust. J. Chem. 1974, 27, 1877–1883.
- Garrett, E.R.; Weber, D.J. Metal Complexes of Thiouracils II: Solubility Analyses and Spectrophotometric Investigations. J. Pharm. Sci. 1971, 60, 845–853.
- Kamalakannan, P.; Venkappayya, D.; Balasubramanian, T. A new antimetabolite, 5-morpholinomethyl-2-thiouracil—Spectral properties, thermal profiles, antibacterial, antifungal and antitumour studies of some of its metal chelates. J. Chem. Soc. Dalton Trans. 2002, 17, 3381–3391.
- Darensbourg, D.J.; Frost, B.J.; Derecskei-Kovacs, A.; Reibenspies, J.H. Coordination Chemistry, Structure, and Reactivity of Thiouracil Derivatives of Tungsten(0) Hexacarbonyl: A Theoretical and Experimental Investigation into the Chelation/Dechelation of Thiouracil via CO Loss and Addition. Inorg. Chem. 1999, 38, 4715–4723.
- Masoud, M.S.; Soayed, A.A.; El-Husseiny, A.F. Coordination modes, spectral, thermal and biological evaluation of hetero-metal copper containing 2-thiouracil complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 365–372.
- Papazoglou, I.; Cox, P.J.; Hatzidimitriou, A.G.; Kokotidou, C.; Choli-Papadopoulou, T.; Aslanidis, P. Copper(I) halide complexes of 5-carbethoxy-2-thiouracil: Synthesis, structure and in vitro cytotoxicity. Eur. J. Med. Chem. 2014, 78, 383–391.
- Kumar, B.; Suman, A. Synthesis, spectroscopic characterization and biological application of copper complex of 5-carbethoxy-2-thiouracil. J. Drug Deliv. Ther. 2020, 10, 145–148.
- Kostova, I. General and inorganic chemistry; Softtrade: Sofia, Bulgaria, 2016; ISBN 978-954-334-185-6.
- Illán-Cabeza, N.A.; García-García, A.R.; Moreno-Carretero, M.N.; Martínez-Martos, J.M.; Ramírez-Expósito, M.J. Synthesis, characterization and antiproliferative behavior of tricarbonyl complexes of rhenium(I) with some 6-amino-5-nitrosouracil derivatives: Crystal structure of fac- (DANU = 6-amino-1,3-dimethyl-5-nitrosouracil). J. Inorg. Biochem. 2005, 99, 1637–1645.
- Abou-Melha, K.S. A Series of Nano-sized Metal ion-thiouracil Complexes, tem, Spectral, γ- irradiation, Molecular Modeling and Biological Studies. Orient. J. Chem. 2015, 31, 1897–1913.
- Golubyatnikova, L.G.; Khisamutdinov, R.A.; Grabovskii, S.A.; Kabal’nova, N.N.; Murinov, Y.I. Complexes of Palladium(II) and Platinum(II) with 6-tert-Butyl-2-thiouracil. Russ. J. Gen.Chem. 2017, 87, 117–121.
- Jayabharathi, J.; Thanikachalam, V.; Jayamoorthy, K.; Perumal, M.V. Computational studies of 1,2-disubstituted benzimidazole derivatives. Spectrochim. Acta A 2012, 97, 6.
- Masoud, M.S.; Amira, M.F.; Ramadan, A.M.; El-Ashry, G.M. Synthesis and characterization of some pyrimidine, purine, amino acid and mixed ligand complexes. Spectrochim. Acta Part A 2008, 69, 230–238.
- Masoud, M.S.; El-Hamid, O.H.A.; Zaki, Z.M. 2-thiouracil-based cobalt(II), nickel(II) and copper(II) complexes. Trans. Met. Chem. 1994, 19, 21–24.
- El-Morsy, F.A.; Jean-Claude, B.J.; Butler, I.S.; El-Sayed, S.A.; Mostafa, S.I. Synthesis, characterization and anticancer activity of new zinc(II), molybdate(II), palladium(II), silver(I), rhodium(III), ruthenium(II) and platinum(II) complexes of 5,6-diamino-4-hydroxy2-mercaptopyrimidine. Inorg. Chim. Acta 2014, 423, 144–155.
- Abás, E.; Pena-Martínez, R.; Aguirre-Ramírez, D.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(I) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49, 1915–1927.
- Holowczak, M.S.; Stancl, M.D.; Wong, G.B. Trichloro( 1-metbylcytosinato)gold(III). Model for DNA interactions. J. Am. Chem. Soc. 1985, 107, 5789–5790.
- Rodriguez, E.C.; Sánchez, J.R.; López-González, J.D.; Salas-Peregrin, J.M.; Olivier, M.J.; Quirós, M.; Beauchamp, A.L. Thermal Behavior and Crystal Structure of Dichlorogold(III). Inorg. Chim. Acta 1990, 171, 151–156.
- Singh, U.P.; Singh, S.; Singh, S.M. Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil. Metal-Based Drugs 1998, 5, 35–39.
- Worachartcheewan, A.; Pingaew, R.; Lekcharoen, D.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, Antioxidant and Antimicrobial Activities of Metal Complexes of 2-thiouracil-hydroxyquinoline Derivatives. Lett. Drug Des. Discov. 2018, 15, 602–611.
This entry is offline, you can click here to edit this entry!
