Targeting the microbiome, microbiota-derived metabolites, and related pathways represents a significant challenge in oncology. Microbiome analyses have confirmed the negative impact of cancer treatment on gut homeostasis, resulting in acute dysbiosis and severe complications, including massive inflammatory immune response, mucosal barrier disruption, and bacterial translocation across the gut epithelium. Moreover, recent studies revealed the relationship between an imbalance in the gut microbiome and treatment-related toxicity. Recently, microbiota modulation via probiotic supplementation and fecal microbiota transplantation represents a new trend in cancer patient care, aiming to increase bacterial diversity, alleviate acute and long-term treatment-induced toxicity, and improve the response to various treatment modalities. A more detailed understanding of the complex relationship between the microbiome and host can significantly contribute to integrating a microbiome-based approach into clinical practice.
- the gut microbiome
- dysbiosis
- cancer treatment efficacy
- late effects
- cognitive impairment
- cardiotoxicity
- probiotics
- fecal microbiota transplantation
1. Introduction
2. Dominant Bacteria-Driven Mechanisms Associated with Cancer Development
2.1. Helicobacter pylori
2.2. Fusobacterium nucleatum
2.3. Escherichia coli
2.4. Salmonella
2.5. Bacteroides fragilis
2.6. Staphylococcus aureus
2.7. Campylobacter jejuni
2.8. Desulfovibrio
2.9. Porphyromonas
3. Microbiome and Treatment Efficacy
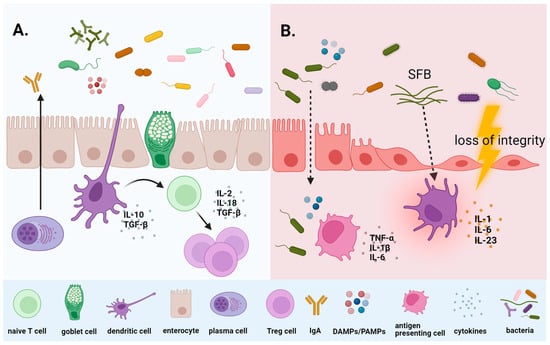
3.1. Microbiome and Chemotherapy
3.2. Microbiome and Immunotherapy
4. Microbiome and Therapy-Induced Late Effects
4.1. Microbiome and Treatment-Induced Cognitive Impairment
4.2. Microbiome and Cardiovascular Toxicity
5. Microbiota Modulation by Probiotics, Prebiotics, and Fecal Microbiota Transplantation in Cancer Patients
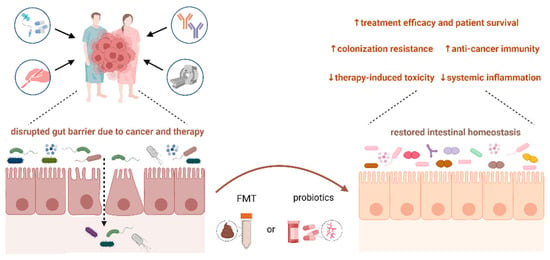
6. Critical Analysis of the Clinical Utility of a Microbiome-Based Approach
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms12010024
References
- Ramirez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Galvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer 2020, 6, 86–97.
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor microbiome—An integral part of the tumor microenvironment. Front. Oncol. 2022, 12, 1063100.
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxid. Med. Cell. Longev. 2016, 2016, 1580967.
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox. Biol. 2021, 42, 101891.
- Ciernikova, S.; Mego, M.; Hainova, K.; Adamcikova, Z.; Stevurkova, V.; Zajac, V. Modification of microflora imbalance: Future directions for prevention and treatment of colorectal cancer? Neoplasma 2015, 62, 345–352.
- Goubet, A.G. Could the tumor-associated microbiota be the new multi-faceted player in the tumor microenvironment? Front. Oncol. 2023, 13, 1185163.
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980.
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490.
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47.
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089.
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 268.
- Narimatsu, H.; Yaguchi, Y.T. The Role of Diet and Nutrition in Cancer: Prevention, Treatment, and Survival. Nutrients 2022, 14, 3329.
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297.
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Diet-driven microbiome changes and physical activity in cancer patients. Front. Nutr. 2023, 10, 1285516.
- Shi, B.; Guo, X.; Liu, H.; Jiang, K.; Liu, L.; Yan, N.; Farag, M.A.; Liu, L. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chem. 2023, 438, 137994.
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649.
- Papadimitriou, N.; Markozannes, G.; Kanellopoulou, A.; Critselis, E.; Alhardan, S.; Karafousia, V.; Kasimis, J.C.; Katsaraki, C.; Papadopoulou, A.; Zografou, M.; et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021, 12, 4579.
- Fontana, A.; Panebianco, C.; Picchianti-Diamanti, A.; Lagana, B.; Cavalieri, D.; Potenza, A.; Pracella, R.; Binda, E.; Copetti, M.; Pazienza, V. Gut Microbiota Profiles Differ among Individuals Depending on Their Region of Origin: An Italian Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 4065.
- Whisner, C.M.; Athena Aktipis, C. The Role of the Microbiome in Cancer Initiation and Progression: How Microbes and Cancer Cells Utilize Excess Energy and Promote One Another’s Growth. Curr. Nutr. Rep. 2019, 8, 42–51.
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615.
- Vidal, M.C.; Anneberg, T.J.; Cure, A.E.; Althoff, D.M.; Segraves, K.A. The variable effects of global change on insect mutualisms. Curr. Opin. Insect Sci. 2021, 47, 46–52.
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344.
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv. Sci. 2023, 10, e2207366.
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977.
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105.
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315.
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78.
- Brown, L.M. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev. 2000, 22, 283–297.
- Chmiela, M.; Kupcinskas, J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. S1), e12638.
- Gonciarz, W.; Walencka, M.; Moran, A.P.; Hinc, K.; Obuchowski, M.; Chmiela, M. Upregulation of MUC5AC production and deposition of LEWIS determinants by HELICOBACTER PYLORI facilitate gastric tissue colonization and the maintenance of infection. J. Biomed. Sci. 2019, 26, 23.
- Noto, J.M.; Peek, R.M., Jr. The Helicobacter pylori cag Pathogenicity Island. Methods Mol. Biol. 2012, 921, 41–50.
- Hanada, K.; Uchida, T.; Tsukamoto, Y.; Watada, M.; Yamaguchi, N.; Yamamoto, K.; Shiota, S.; Moriyama, M.; Graham, D.Y.; Yamaoka, Y. Helicobacter pylori infection introduces DNA double-strand breaks in host cells. Infect. Immun. 2014, 82, 4182–4189.
- Backert, S.; Tegtmeyer, N.; Fischer, W. Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Future Microbiol. 2015, 10, 955–965.
- Saadat, I.; Higashi, H.; Obuse, C.; Umeda, M.; Murata-Kamiya, N.; Saito, Y.; Lu, H.; Ohnishi, N.; Azuma, T.; Suzuki, A.; et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 2007, 447, 330–333.
- Sun, C.H.; Li, B.B.; Wang, B.; Zhao, J.; Zhang, X.Y.; Li, T.T.; Li, W.B.; Tang, D.; Qiu, M.J.; Wang, X.C.; et al. The role of Fusobacterium nucleatum in colorectal cancer: From carcinogenesis to clinical management. Chronic Dis. Transl. Med. 2019, 5, 178–187.
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306.
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298.
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Ziebolz, D.; Schmalz, G.; Jia, B.; Zhao, J. More Than Just a Periodontal Pathogen-the Research Progress on Fusobacterium nucleatum. Front. Cell. Infect. Microbiol. 2022, 12, 815318.
- Liu, H.; Liu, Y.; Liu, W.; Zhang, W.; Xu, J. EZH2-mediated loss of miR-622 determines CXCR4 activation in hepatocellular carcinoma. Nat. Commun. 2015, 6, 8494.
- Gaba, F.I.; Gonzalez, R.C.; Martinez, R.G. The Role of Oral Fusobacterium nucleatum in Female Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Dent. 2022, 2022, 1876275.
- Han, Y.W.; Ikegami, A.; Rajanna, C.; Kawsar, H.I.; Zhou, Y.; Li, M.; Sojar, H.T.; Genco, R.J.; Kuramitsu, H.K.; Deng, C.X. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 2005, 187, 5330–5340.
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206.
- Guo, P.; Tian, Z.; Kong, X.; Yang, L.; Shan, X.; Dong, B.; Ding, X.; Jing, X.; Jiang, C.; Jiang, N.; et al. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 2020, 39, 202.
- Magdy, A.; Elhadidy, M.; Abd Ellatif, M.E.; El Nakeeb, A.; Abdallah, E.; Thabet, W.; Youssef, M.; Khafagy, W.; Morshed, M.; Farid, M. Enteropathogenic Escherichia coli (EPEC): Does it have a role in colorectal tumourigenesis? A Prospective Cohort Study. Int. J. Surg. 2015, 18, 169–173.
- Villeger, R.; Lopes, A.; Veziant, J.; Gagniere, J.; Barnich, N.; Billard, E.; Boucher, D.; Bonnet, M. Microbial markers in colorectal cancer detection and/or prognosis. World J. Gastroenterol. 2018, 24, 2327–2347.
- Scott, D.A.; Kaper, J.B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 1994, 62, 244–251.
- Tremblay, W.; Mompart, F.; Lopez, E.; Quaranta, M.; Bergoglio, V.; Hashim, S.; Bonnet, D.; Alric, L.; Mas, E.; Trouche, D.; et al. Cytolethal Distending Toxin Promotes Replicative Stress Leading to Genetic Instability Transmitted to Daughter Cells. Front. Cell Dev. Biol. 2021, 9, 656795.
- Sepe, L.P.; Hartl, K.; Iftekhar, A.; Berger, H.; Kumar, N.; Goosmann, C.; Chopra, S.; Schmidt, S.C.; Gurumurthy, R.K.; Meyer, T.F.; et al. Genotoxic Effect of Salmonella Paratyphi A Infection on Human Primary Gallbladder Cells. mBio 2020, 11, e01911-20.
- Duijster, J.W.; Hansen, J.V.; Franz, E.; Neefjes, J.J.C.; Frisch, M.; Mughini-Gras, L.; Ethelberg, S. Association between Salmonella infection and colon cancer: A nationwide registry-based cohort study. Epidemiol. Infect. 2021, 149, e56.
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; Pelt, W.V.; Neefjes, J. Increased colon cancer risk after severe Salmonella infection. PLoS ONE 2018, 13, e0189721.
- Lu, R.; Wu, S.; Zhang, Y.G.; Xia, Y.; Zhou, Z.; Kato, I.; Dong, H.; Bissonnette, M.; Sun, J. Salmonella Protein AvrA Activates the STAT3 Signaling Pathway in Colon Cancer. Neoplasia 2016, 18, 307–316.
- Liu, X.; Lu, R.; Xia, Y.; Wu, S.; Sun, J. Eukaryotic signaling pathways targeted by Salmonella effector protein AvrA in intestinal infection in vivo. BMC Microbiol. 2010, 10, 326.
- Wu, H.; Jones, R.M.; Neish, A.S. The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell. Microbiol. 2012, 14, 28–39.
- Viljoen, K.S.; Dakshinamurthy, A.; Goldberg, P.; Blackburn, J.M. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS ONE 2015, 10, e0119462.
- Pierce, J.V.; Bernstein, H.D. Genomic Diversity of Enterotoxigenic Strains of Bacteroides fragilis. PLoS ONE 2016, 11, e0158171.
- Yu, L.C.; Wei, S.C.; Ni, Y.H. Impact of microbiota in colorectal carcinogenesis: Lessons from experimental models. Intest. Res. 2018, 16, 346–357.
- Bundgaard-Nielsen, C.; Baandrup, U.T.; Nielsen, L.P.; Sorensen, S. The presence of bacteria varies between colorectal adenocarcinomas, precursor lesions and non-malignant tissue. BMC Cancer 2019, 19, 399.
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197.
- Espersen, F.; Frimodt-Moller, N.; Rosdahl, V.T.; Jessen, O.; Faber, V.; Rosendal, K. Staphylococcus aureus bacteremia in patients with hematological malignancies and/or agranulocytosis. Acta Medica Scand 1987, 222, 465–470.
- Velasco, E.; Thuler, L.C.; Martins, C.A.; Nucci, M.; Dias, L.M.; Goncalves, V.M. Epidemiology of bloodstream infections at a cancer center. Sao Paulo Med. J. 2000, 118, 131–138.
- Turk, S.; Yanpar, H.; Baesmat, A.S.; Canli, S.D.; Cinar, O.E.; Malkan, U.Y.; Turk, C.; Haznedaroglu, I.C.; Ucar, G. Enterotoxins A and B produced by Staphylococcus aureus increase cell proliferation, invasion and cytarabine resistance in acute myeloid leukemia cell lines. Heliyon 2023, 9, e19743.
- Akbari, A.; Farahnejad, Z.; Akhtari, J.; Abastabar, M.; Mobini, G.R.; Mehbod, A.S. Staphylococcus aureus Enterotoxin B Down-Regulates the Expression of Transforming Growth Factor-Beta (TGF-beta) Signaling Transducers in Human Glioblastoma. Jundishapur J. Microbiol. 2016, 9, e27297.
- Sun, X.; Threadgill, D.; Jobin, C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology 2012, 142, 86–95.e5.
- Konkel, M.E.; Talukdar, P.K.; Negretti, N.M.; Klappenbach, C.M. Taking Control: Campylobacter jejuni Binding to Fibronectin Sets the Stage for Cellular Adherence and Invasion. Front. Microbiol. 2020, 11, 564.
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006, 4, 9–14.
- Dahmus, J.D.; Kotler, D.L.; Kastenberg, D.M.; Kistler, C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. J. Gastrointest. Oncol. 2018, 9, 769–777.
- Kapral, M.; Weglarz, L.; Parfiniewicz, B.; Lodowska, J.; Jaworska-Kik, M. Quantitative evaluation of transcriptional activation of NF-kappaB p65 and p50 subunits and IkappaBalpha encoding genes in colon cancer cells by Desulfovibrio desulfuricans endotoxin. Folia Microbiol. 2010, 55, 657–661.
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127.
- Olsen, I.; Yilmaz, O. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral Microbiol. 2019, 11, 1563410.
- Zhou, L.; Dorfer, C.E.; Chen, L.; Fawzy El-Sayed, K.M. Porphyromonas gingivalis lipopolysaccharides affect gingival stem/progenitor cells attributes through NF-kappaB, but not Wnt/beta-catenin, pathway. J. Clin. Periodontol. 2017, 44, 1112–1122.
- Al-Attar, A.; Alimova, Y.; Kirakodu, S.; Kozal, A.; Novak, M.J.; Stromberg, A.J.; Orraca, L.; Gonzalez-Martinez, J.; Martinez, M.; Ebersole, J.L.; et al. Activation of Notch-1 in oral epithelial cells by P. gingivalis triggers the expression of the antimicrobial protein PLA(2)-IIA. Mucosal Immunol. 2018, 11, 1047–1059.
- Burotto, M.; Wilkerson, J.; Stein, W.D.; Bates, S.E.; Fojo, T. Adjuvant and neoadjuvant cancer therapies: A historical review and a rational approach to understand outcomes. Semin. Oncol. 2019, 46, 83–99.
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281.
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22.
- Einhorn, L.H. Treatment of testicular cancer: A new and improved model. J. Clin. Oncol. 1990, 8, 1777–1781.
- Poletto, S.; Novo, M.; Paruzzo, L.; Frascione, P.M.M.; Vitolo, U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat. Rev. 2022, 110, 102443.
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401.
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976.
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160.
- Tsai, H.F.; Hsu, P.N. Cancer immunotherapy by targeting immune checkpoints: Mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J. Biomed. Sci. 2017, 24, 35.
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267.
- Reckova, M.; Mladosievicova, B. An ongoing evolution of cardio-oncology with the rapid development of modern immunotherapy. Int. J. Cardiol. 2022, 347, 60–61.
- Zomborska, E.; Kasperova, S.; Slopovsky, J.; Pazderova, N.; Kasperova, B.; Penz, P.; Nyitrayova, O.; Salek, T.; Porsok, S.; Mladosievicova, B.; et al. Fatal myocarditis after the first dose of nivolumab. Klin. Onkol. 2022, 35, 486–492.
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435.
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47.
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249.
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021, 33, 988–1000.e7.
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602.
- Ahmad, S.S.; Reinius, M.A.; Hatcher, H.M.; Ajithkumar, T.V. Anticancer chemotherapy in teenagers and young adults: Managing long term side effects. BMJ 2016, 354, i4567.
- Bajic, J.E.; Johnston, I.N.; Howarth, G.S.; Hutchinson, M.R. From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation. Front. Behav. Neurosci. 2018, 12, 104.
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157.
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478.
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133.
- Kong, C.; Liang, L.; Liu, G.; Du, L.; Yang, Y.; Liu, J.; Shi, D.; Li, X.; Ma, Y. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 2023, 72, 1129–1142.
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared with Age-Matched Controls: An Analysis from a Nationwide, Multicenter, Prospective Longitudinal Study. J. Clin. Oncol. 2017, 35, 506–514.
- Chovanec, M.; Vasilkova, L.; Setteyova, L.; Obertova, J.; Palacka, P.; Rejlekova, K.; Sycova-Mila, Z.; Kalavska, K.; Svetlovska, D.; Cingelova, S.; et al. Long-Term Cognitive Functioning in Testicular Germ-Cell Tumor Survivors. Oncologist 2018, 23, 617–623.
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361.
- Couch, L.S.; Lyon, A.R.; Lopez-Fernandez, T. Cardio-oncology: A new field requiring guidance. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e47.
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311.
- Mladosievicova, B.; Petrikova, L.; Valaskova, Z.; Bernadic, M., Jr.; Chovanec, M.; Mego, M.; Bernadic, M., Sr. Atherosclerosis in cancer patients. Bratisl. Lek. Listy 2019, 120, 636–640.
- Mertens, A.C.; Yasui, Y.; Neglia, J.P.; Potter, J.D.; Nesbit, M.E., Jr.; Ruccione, K.; Smithson, W.A.; Robison, L.L. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J. Clin. Oncol. 2001, 19, 3163–3172.
- Grilz, E.; Posch, F.; Nopp, S.; Konigsbrugge, O.; Lang, I.M.; Klimek, P.; Thurner, S.; Pabinger, I.; Ay, C. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer-a nationwide analysis. Eur. Heart J. 2021, 42, 2299–2307.
- Florido, R.; Daya, N.R.; Ndumele, C.E.; Koton, S.; Russell, S.D.; Prizment, A.; Blumenthal, R.S.; Matsushita, K.; Mok, Y.; Felix, A.S.; et al. Cardiovascular Disease Risk among Cancer Survivors: The Atherosclerosis Risk in Communities (ARIC) Study. J. Am. Coll. Cardiol. 2022, 80, 22–32.
- Herrema, H.; Nieuwdorp, M.; Groen, A.K. Microbiome and Cardiovascular Disease. In Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 311–334.
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673.
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211.
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947.
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326.
- Washburn, R.L.; Cox, J.E.; Muhlestein, J.B.; May, H.T.; Carlquist, J.F.; Le, V.T.; Anderson, J.L.; Horne, B.D. Pilot Study of Novel Intermittent Fasting Effects on Metabolomic and Trimethylamine N-oxide Changes During 24-hour Water-Only Fasting in the FEELGOOD Trial. Nutrients 2019, 11, 246.
- Ciernikova, S.; Sevcikova, A.; Drgona, L.; Mego, M. Modulating the gut microbiota by probiotics, prebiotics, postbiotics, and fecal microbiota transplantation: An emerging trend in cancer patient care. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188990.
- Huang, F.; Li, S.; Chen, W.; Han, Y.; Yao, Y.; Yang, L.; Li, Q.; Xiao, Q.; Wei, J.; Liu, Z.; et al. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients 2023, 15, 356.
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362.
- Mego, M.; Danis, R.; Chovanec, J.; Jurisova, S.; Bystricky, B.; Porsok, S.; Konkolovsky, P.; Vaclav, V.; Wagnerova, M.; Stresko, M.; et al. Randomized double-blind, placebo-controlled multicenter phase III study of prevention of irinotecan-induced diarrhea by a probiotic mixture containing Bifidobacterium BB-12((R))Lactobacillus rhamnosus LGG((R)) in colorectal cancer patients. Front. Oncol. 2023, 13, 1168654.
- Cui, M.; Xiao, H.; Li, Y.; Zhou, L.; Zhao, S.; Luo, D.; Zheng, Q.; Dong, J.; Zhao, Y.; Zhang, X.; et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017, 9, 448–461.
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103.
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97.
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schonlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168–174.
