Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Circulating tumor cells (CTCs) are cells released from the primary and metastatic tumor and intravasate into the blood or lymphatic vessels, where they are transported to distant sites and act as seeds that initiate cancer metastases or the development of further lesions.
- circulating tumor cells
- liquid biopsy
- cancer research
- metastasis
- personalized medicine
1. Introduction
In the United States (US), cancer remains the second leading cause of death, affecting individuals of all ages and ethnic groups [1]. The estimated number of all cancer-related deaths in the US is over 609,000, which is about 18% of all US deaths [1]. Cancer mortality is due to poor treatment response, metastasis, and the lack of early detection methods [2,3,4]. Liquid biopsies are gaining popularity as noninvasive techniques for early disease detection [5,6]. These liquid biopsies contain circulating tumor cells (CTCs) that are released into the blood by a tumor and travel through the bloodstream to other areas of the body to form metastatic niches (Figure 1) [7].
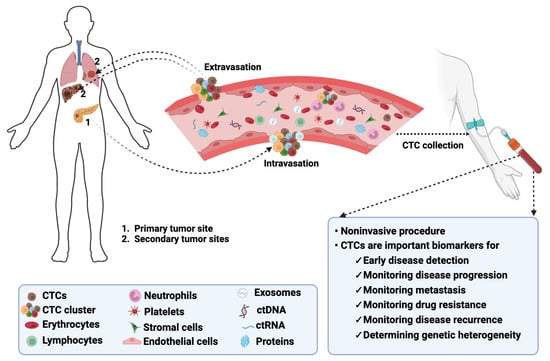
Figure 1. Biology of CTCs in cancer. CTCs detach from their primary tumors, intravasate into the bloodstream, and extravasate from the bloodstream to colonize secondary tumors or metastatic sites. CTCs can be detected following noninvasive collection procedures and serve as biomarkers for monitoring multiple disease aspects. The image was created using BioRender.com.
CTCs are proving to be essential biomarkers that can be used for early disease detection, monitoring metastasis and cancer progression, and determining genetic heterogeneity in tumors [8,9,10]. Compared to traditional tumor biopsies, obtaining CTCs from liquid biopsies is non-invasive and real-time [11]. This can be more acceptable to patients, paving the way for more personalized cancer medicine.
However, several challenges currently affect CTC research. One of the main issues is that they are rare and heterogeneous [12,13]. CTCs are present in very small numbers, about 1–100 per 109 blood cells [14]. These scarcely populated cells are characterized by varying morphology, phenotypical characteristics, and genetic makeup, making isolation and characterization difficult [15,16]. Moreover, standardizing isolation techniques and establishing their clinical relevance presents ongoing challenges [17,18]. Ethical considerations and the need for more extensive clinical trials also pose significant obstacles to advancing CTC research [19]. Available techniques for detecting CTCs, like flow cytometry-based and microscopy-based detection methods, need refinements to improve accuracy and sensitivity [20].
Current approaches to the isolation and analysis of CTC biology involve using microfluidic devices, single-cell sequencing, and the integration of multi-omics data to determine the molecular and functional heterogeneity of CTCs [21,22,23]. Machine learning tools trained to interpret complex CTC data are also being developed to aid in identifying biomarkers and potential therapeutic targets [24,25]. These methods hold promise for improved detection, monitoring, and uncovering treatment-resistant or metastatic CTC subpopulations.
2. Significance of CTCs in Cancer and Clinical Implications
2.1. Early Cancer Detection
Early detection of cancer is crucial for effective treatment and disease management to reduce cancer-related deaths and improve patient outcomes. CTCs offer the potential for liquid biopsy-based screening, allowing the identification of cancers at an earlier, more treatable stage [26]. CTCs were first detected in cancer in the eighteenth century [27]. However, the process of local invasion and extravasation of CTCs has recently been shown to occur within a short span of time [28], suggesting that CTCs can be detected in the bloodstream at early stages of cancer, potentially allowing for early diagnosis before tumors become clinically evident [29,30]. A study involving 667 participants, of whom 235 were healthy individuals and 432 were patients with either colorectal cancer (CRC) or adenomas, showed that CTCs can be used to distinguish healthy individuals from patients with CRC or adenomas with a detection sensitivity of up to 95.2%, even before colonoscopy [31]. Another study evaluating patients with chronic obstructive pulmonary disease (COPD) without clinically diagnosed lung cancer revealed that five out of 168 patients had detectable CTCs. Notably, all five individuals with detectable CTCs developed lung nodules within 1–4 years [32]. A third study involving patients with suspected prostate cancer based on high serum prostate-specific antigen (PSA) levels also revealed a high positive CTC-predicted biopsy outcome and prostate cancer aggressiveness [33]. Thus, the importance of using CTCs to predict disease occurrence cannot be underestimated. Additionally, capturing and analyzing CTCs using liquid biopsy techniques offer a non-invasive alternative to traditional tissue biopsies, providing a real-time snapshot of the tumor’s genetic and phenotypic characteristics.
2.2. Prognostic Indicators
The presence of CTCs in the bloodstream provides valuable prognostic information, aiding in predicting disease outcomes and guiding treatment decisions [34,35,36]. Studies have shown that higher CTC counts and the presence of CTC clusters are associated with poorer outcomes across various cancer types [37,38]. In clinical settings, CTCs are commonly used as a liquid biopsy method to screen for tumors, monitor disease, and predict patients’ prognoses [39]. A CTC count cutoff value of ≥ 3 CTCs per 7.5 mL blood is considered unfavorable for metastatic colorectal cancer [40]. In a study involving hormone receptor-positive (HR+) metastatic breast cancer patients, the overall survival (OS) and progression-free survival (PFS) of patients with CTC counts ≥ 5 per 7.5 mL blood after treatment was significantly worse than those of patients with < 5 CTCs [41]. Also, CTC counts ≥ 10 per 5 mL blood in small-cell lung cancer (SCLC) patients were closely associated with advanced stage (high lymph node metastasis and distant metastasis), indicating a more unfavorable prognosis [42]. These recent investigations have refined the understanding of CTCs as robust prognostic indicators, guiding clinicians in predicting disease outcomes and tailoring treatment strategies. Multiple detection methods have been developed to comprehensively characterize CTCs, providing valuable insights into the likely cause of disease [39,43]. CTC enumeration is increasingly being incorporated into clinical practice as a predictive tool, and CTC counts are being used to stratify patients, helping tailor treatment plans based on individual prognoses [44].
2.3. Tumor Heterogeneity
Tumor cells obtained from liquid biopsies exhibit genetic and phenotypic diversity, reflecting heterogeneity within the primary tumor [13,45]. Numerous studies have discovered the presence of different CTC subpopulations in cancers [13,46,47,48]. For instance, Freed and colleagues identified two CTC subpopulations expressing epithelial cell adhesion molecule, EpCAM (CTCEpCAM), or fibroblast activation protein-alpha, FAPα (CTCFAPα), in pancreatic ductal adenocarcinoma (PDAC) patients. Using the ratios of CTCFAPα to CTCEpCAM, they were able to stratify patients as responders versus non-responders to niraparib treatment [46]. In a non-small cell lung cancer (NSCLC) study, the authors were able to detect different gene signatures related to therapy resistance (MET and HER3) and the initiation of metastasis (ALHD1) using CTCs [49]. The spectrum of cancer heterogeneity has long been under scrutiny as a factor allowing the tumor to adapt to different microenvironmental stressors with different subpopulations that may evolve to increase disease aggressiveness and decrease therapeutic response [50]. The study of CTCs, therefore, allows researchers to understand and monitor tumor heterogeneity, providing crucial information for devising effective and personalized treatment strategies.
2.4. Metastasis and Disease Progression
Metastasis accounts for more than 90% of cancer-related mortalities [51,52,53]. This is because treatment options available for patients with metastatic solid tumors are rarely curative [54,55]. Metastatic events in cancer involve the spread of tumor cells from the primary to secondary sites, following a phenotypic transition from epithelial to mesenchymal phenotype, cancer cell invasion into circulation, dormancy, and colonization at distant sites [56,57]. CTCs can be released by aggressive and metastatic tumors into circulation, where they extravasate to other remote sites for continuous colonization, leading to the growth of further lesions [58]. Nonetheless, research suggests that CTCs can be shed from primary tumors at early stages of cancer, challenging the traditional view that metastasis occurs primarily in advanced disease [6]. This early dissemination contributes to the understanding of metastatic potential and the dynamics of cancer progression [6,59]. Following the injection of pancreatic cancer cells expressing fluorescent proteins into the earlobes of mice to form solid tumors, the presence of CTCs with fluorescent proteins was detected in the bloodstream of the mice at different stages of development [60]. Using quantum dots, the researchers identified cells with high metastatic potential; those expressing clusters of differentiation, CD24+ and CD133+ [60]. The development of metastatic niches often signifies a significant progression in the tumor stage, and studies have linked CTC characteristics, such as the presence of CTC clusters, to increased metastatic potential, aiding in the identification of patients at higher risk [61,62,63]. In metastatic breast cancer, a CTC count ≥ 5 in 7.5 mL of blood correlates with worse overall survival and progression-free survival [40]. Indeed, a CTC count of less than five in patients with stage IV breast cancer is used to classify the tumor as stage IV indolent, whereas a CTC count greater than five classifies the tumor as stage IV aggressive [40]. CTC clusters exhibit stemness characteristics and can evade the immune system by recruiting immunosuppressive cells [64]. This attribute helps prevent CTCs from being attacked by antitumor immune cells like natural killer (NK) cells, thereby increasing their metastatic potential [64,65,66]. In circulation, CTCs are protected from shear forces and shielded from immune detection through interactions with platelets [67]. Other blood cells, like macrophages, are able to interact directly with CTCs to protect them from being phagocytosed [67]. Szczerba et al. also identified and associated CTC–neutrophil interactions with cell cycle progression, leading to more efficient metastasis formation [68]. Using a mass spectrometry-based untargeted metabolomics approach, human colorectal cancer CTC-derived cells were shown to have low or high metastatic potential based on metabolic features [69]. A combination of metabolites, such as glutamic acid, malic acid, lactic acid, and aspartic acid, along with higher CTC counts, positively predicted the metastatic risk in patients, providing evidence of the influence of metabolic phenotype on the metastatic potential of cells [69]. Recently, CTCs were shown to have unexpectedly high levels of OXPHOS compared to glycolytic signatures [70]. This metabolic reprogramming was the opposite of the common “Warburg Effect” seen in metastatic cancer cells, suggesting an additional layer of regulative complexities in cancer metastasis [70]. Metabolic reprogramming of CTCs enables them to survive harsh conditions in the bloodstream and enhances their ability to establish a favorable microenvironment for metastasis, known as metastatic niches [71]. The molecular mechanisms by which CTCs influence the pre-metastatic niche (PMN), prepare distant organs for colonization, and contribute to the metastatic process are yet to be fully understood. However, CTCs acquire enhanced migratory and invasive capabilities when undergoing epithelial–mesenchymal transition (EMT) [72]. Recent discoveries have elucidated the dynamic nature of EMT in CTCs, identifying hybrid epithelial/mesenchymal states that may contribute to metastatic progression [38]. For instance, the presence of both epithelial and mesenchymal markers indicates the occurrence of intrahepatic metastasis, while the presence of mesenchymal phenotypes prompts the development of extrahepatic metastasis [38]. Expression of mesenchymal markers like N-cadherin, vimentin, snail, and slug have also been detected in CTCs, highlighting their use in metastasis prediction [73,74]. Using CTC-based information to assess the risk of metastasis will help implement more aggressive treatment strategies for patients with a higher likelihood of developing metastatic disease.
2.5. Treatment Response Monitoring
Changes in CTC counts and characteristics during treatment can indicate treatment response or resistance [41]. Real-time monitoring of CTC dynamics will allow for timely adjustments to therapeutic strategies. To monitor patient response to therapy, longitudinal tissue biopsies must be collected. However, this procedure is often invasive and complicated by the location and size of tumors [75]. Therefore, CTCs can serve as dynamic biomarkers for monitoring treatment response and assessing the efficacy of therapies. Indeed, CTCs from patients with either chemosensitive or chemorefractory SCLC were tumorigenic in immune-compromised mice, and their resultant CTC-derived explants mirrored the donor patients’ response to platinum and etoposide chemotherapy [76]. Molecular alterations that affect a tumor cell’s response to specific drugs can also be determined through molecular profiling of CTCs. For example, the expression level of cyclin D1 (CCND1) was shown to be significantly reduced in patients with head and neck squamous cell carcinoma (HNSCC) following nivolumab treatment [77]. In contrast, the expression of NANOG increased significantly [77]. Changes in CTC count or specific genetic alterations can, thus, indicate whether a treatment effectively targets the tumor, enabling timely adjustments to the therapeutic approach.
2.6. Minimal Residual Disease Monitoring
CTCs can be used to monitor minimal residual disease (MRD) after surgery or other treatments, helping identify potential disease recurrence early. Li and colleagues used preoperative CTC concentration to predict postoperative recurrence or metastasis in patients with NSCLC [78]. Detection of CTCs pre- and post-adjuvant chemotherapy can also serve as prognostic indicators of early relapse [34]. These indicators allow for investigations into novel therapeutic approaches to curb relapse and drug resistance. CTC-based MRD monitoring has also been explored as a valuable tool in post-treatment surveillance, offering insights into the effectiveness of interventions and informing decisions regarding additional therapies [79]. CTCs can enter a state of dormancy, temporarily evading the immune system and therapeutic interventions [80,81]. Recent studies have shed light on the mechanisms governing CTC dormancy and the factors that lead to their reactivation, contributing to a deeper understanding of cancer relapse [82,83,84]. Molecular characterization of CTCs can also help identify and analyze drug-tolerant clones within the tumor microenvironment (TME) [85].
2.7. Personalized Medicine
Precision oncology aims to treat patients according to the molecular characteristics of their tumors, adjusting these treatment strategies as the tumors evolve [86]. Molecular characterization of CTCs contributes to identifying actionable mutations and potential therapeutic targets. Whole exome sequencing (WES) of patient CTC samples has been shown to reflect the genomic characteristics of their corresponding solid tumors more accurately [87,88,89]. This genomic information can be crucial in developing personalized treatment plans. Functional mutations in driver genes like EGFR, KRAS, and TP53 can be determined quickly and used to stratify patients for therapy [89]. Mutational characterization of CTCs can also help physicians decipher if and when a patient is developing resistance to a particular chemotherapeutic agent, thereby allowing for proactive treatment decisions [90]. In this regard, CTC-based liquid biopsies facilitate the development of personalized treatment plans, allowing for the selection of targeted therapies based on the specific genetic profile of the tumor. Furthermore, CTC-based liquid biopsies are increasingly integrated into clinical trials and treatment decision-making.
2.8. Clinical Trials and Drug Development
CTCs offer a tool for assessing drug response in preclinical and clinical trials, aiding in developing novel cancer therapeutics. Current CTC-based clinical trials aim to use CTC positivity and dynamics to predict clinical outcomes and therapy responses in patients [19]. Following the STIC CTC randomized clinical trial (NCT0170605), researchers determined that CTC count was a reliable biomarker for choosing between chemotherapy and a single-agent endocrine therapy as a first-line treatment in hormone receptor-positive ERBB2-negative metastatic breast cancer [91]. Data from clinical trials can also be used to model the prognostic impact of a tumor and the design of specific targeting agents [92]. Therefore, CTC-based studies provide a platform for evaluating the effectiveness of new treatments and understanding drug resistance mechanisms.
In summary, CTCs have a significant role in cancer research and clinical applications. They act as dynamic biomarkers, providing insights into the complex nature of cancer. With technological advancements, CTC-based approaches can be integrated into regular clinical practice. This can potentially revolutionize cancer diagnosis, treatment, and patient outcomes.
This entry is adapted from the peer-reviewed paper 10.3390/cancers16061213
This entry is offline, you can click here to edit this entry!
