Despite improvements in contemporary medical and surgical therapies, cardiovascular disease (CVD) remains a significant cause of worldwide morbidity and mortality; more specifically, ischemic heart disease (IHD) may affect individuals as young as 20 years old. Typically managed with guideline-directed medical therapy, interventional or surgical methods, the incurred cardiomyocyte loss is not always completely reversible; however, recent research into various stem cell (SC) populations has highlighted their potential for the treatment and perhaps regeneration of injured cardiac tissue, either directly through cellular replacement or indirectly through local paracrine effects. Different stem cell (SC) types have been employed in studies of infarcted myocardium, both in animal models of myocardial infarction (MI) as well as in clinical studies of MI patients, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), Muse cells, multipotent stem cells such as bone marrow-derived cells, mesenchymal stem cells (MSCs) and cardiac stem and progenitor cells (CSC/CPCs). These have been delivered as is, in the form of cell therapies, or have been used to generate tissue-engineered (TE) constructs with variable results.
- cardiovascular disease
- coronary artery disease
- myocardial infarction
- cardiac surgery
1. Cell Therapies
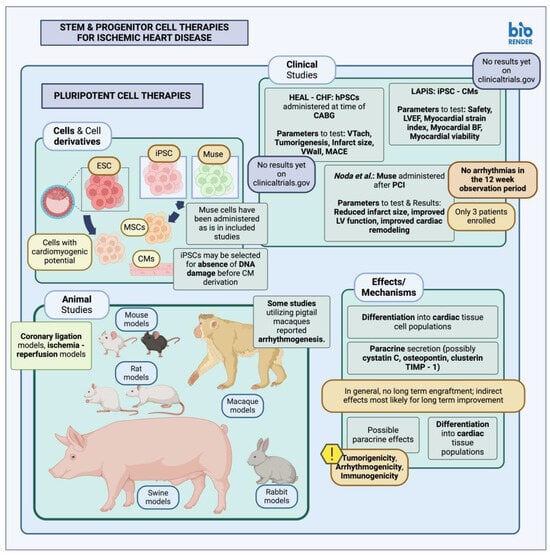
1.1. Pluripotent Stem Cells in Animal Studies and Clinical Trials
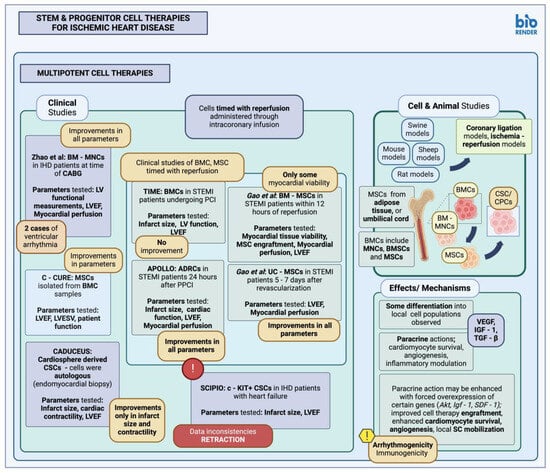
1.2. Multipotent Stem Cells in Animal Studies and Clinical Trials
2. Tissue-Engineered Therapies
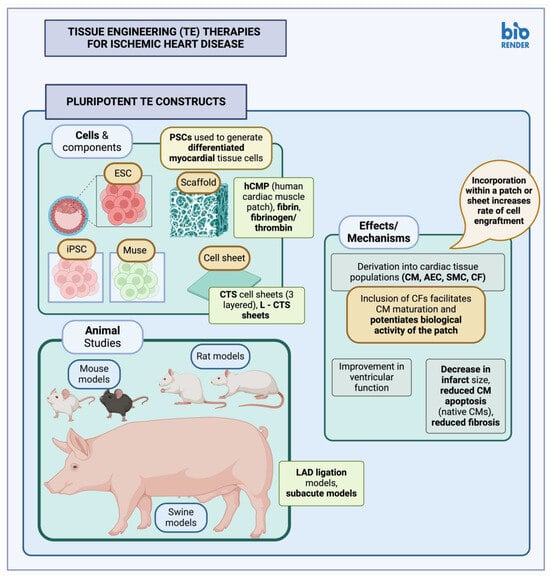
2.1. Pluripotent Stem Cell Constructs
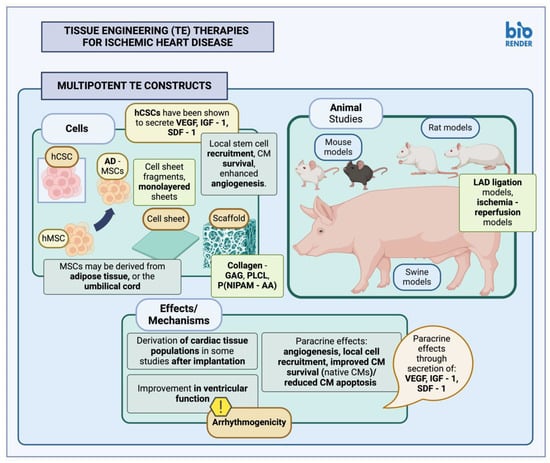
2.2. Multipotent Stem Cell Constructs
This entry is adapted from the peer-reviewed paper 10.3390/cimb46030141
References
- Abu-Dawud, R.; Graffmann, N.; Ferber, S.; Wruck, W.; Adjaye, J. Pluripotent Stem Cells: Induction and Self-Renewal. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170213.
- Singla, D.K.; Lyons, G.E.; Kamp, T.J. Transplanted Embryonic Stem Cells Following Mouse Myocardial Infarction Inhibit Apoptosis and Cardiac Remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1308–H1314.
- Min, J.-Y.; Yang, Y.; Sullivan, M.F.; Ke, Q.; Converso, K.L.; Chen, Y.; Morgan, J.P.; Xiao, Y.-F. Long-Term Improvement of Cardiac Function in Rats after Infarction by Transplantation of Embryonic Stem Cells. J. Thorac. Cardiovasc. Surg. 2003, 125, 361–369.
- Help Therapeutics Epicardial Injection of Allogeneic Human Pluripotent Stem Cell-Derived Cardiomyocytes to Treat Severe Chronic Heart Failure. 2022. Available online: https://clinicaltrials.gov/study/NCT03763136#publications (accessed on 25 December 2023).
- Heartseed Inc. A Phase I/II Study of Human Induced Pluripotent Stem (iPS) Cell-Derived Cardiomyocyte Spheroids (HS-001) in Patients with Severe Heart Failure, Secondary to Ischemic Heart Disease. 2022. Available online: https://clinicaltrials.gov/study/NCT04945018 (accessed on 25 December 2023).
- Noda, T.; Nishigaki, K.; Minatoguchi, S. Safety and Efficacy of Human Muse Cell-Based Product for Acute Myocardial Infarction in a First-in-Human Trial. Circ. J. 2020, 84, 1189–1192.
- Soma, Y.; Tani, H.; Morita-Umei, Y.; Kishino, Y.; Fukuda, K.; Tohyama, S. Pluripotent Stem Cell-Based Cardiac Regenerative Therapy for Heart Failure. J. Mol. Cell Cardiol. 2024, 187, 90–100.
- Hatani, T.; Yoshida, Y. Transplantation of Human Induced Pluripotent Stem Cell-Derived CardiomyocytesHuman Induced Pluripotent Stem Cell-Derived Cardiomyocytes (iPSC-CMs) in a Mouse Myocardial InfarctionMyocardial Infarction Model. In Pluripotent Stem-Cell Derived Cardiomyocytes; Yoshida, Y., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 285–293. ISBN 978-1-07-161484-6.
- Thavapalachandran, S.; Le, T.Y.L.; Romanazzo, S.; Rashid, F.N.; Ogawa, M.; Kilian, K.A.; Brown, P.; Pouliopoulos, J.; Barry, A.M.; Fahmy, P.; et al. Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Improve Cardiac Function and Vascularity after Myocardial Infarction. Cytotherapy 2021, 23, 1074–1084.
- Kannappan, R.; Turner, J.F.; Miller, J.M.; Fan, C.; Rushdi, A.G.; Rajasekaran, N.S.; Zhang, J. Functionally Competent DNA Damage-Free Induced Pluripotent Stem Cell–Derived Cardiomyocytes for Myocardial Repair. Circulation 2019, 140, 520–522.
- Kobayashi, H.; Ichimura, H.; Ohashi, N.; Shiba, Y. Transplantation of Pluripotent Stem Cell-Derived Cardiomyocytes into a Myocardial Infarction Model of Cynomolgus Monkey. Methods Mol. Biol. 2021, 2320, 295–302.
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature 2014, 510, 273–277.
- Higuchi, A.; Ku, N.-J.; Tseng, Y.-C.; Pan, C.-H.; Li, H.-F.; Kumar, S.S.; Ling, Q.-D.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; et al. Stem Cell Therapies for Myocardial Infarction in Clinical Trials: Bioengineering and Biomaterial Aspects. Lab. Investig. 2017, 97, 1167–1179.
- Grimaldi, V.; Mancini, F.P.; Casamassimi, A.; Al-Omran, M.; Zullo, A.; Infante, T.; Napoli, C. Potential Benefits of Cell Therapy in Coronary Heart Disease. J. Cardiol. 2013, 62, 267–276.
- Selvakumar, D.; Reyes, L.; Chong, J.J.H. Cardiac Cell Therapy with Pluripotent Stem Cell-Derived Cardiomyocytes: What Has Been Done and What Remains to Do? Curr. Cardiol. Rep. 2022, 24, 445–461.
- Alanazi, R.F.; Alhwity, B.S.; Almahlawi, R.M.; Alatawi, B.D.; Albalawi, S.A.; Albalawi, R.A.; Albalawi, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells 2023, 12, 1676.
- Ogawa, E.; Oguma, Y.; Kushida, Y.; Wakao, S.; Okawa, K.; Dezawa, M. Naïve Pluripotent-like Characteristics of Non-Tumorigenic Muse Cells Isolated from Human Amniotic Membrane. Sci. Rep. 2022, 12, 17222.
- Yamada, Y.; Wakao, S.; Kushida, Y.; Minatoguchi, S.; Mikami, A.; Higashi, K.; Baba, S.; Shigemoto, T.; Kuroda, Y.; Kanamori, H.; et al. S1P–S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ. Res. 2018, 122, 1069–1083.
- Ratajczak, M.Z.; Suszynska, M.; Borkowska, S.; Ratajczak, J.; Schneider, G. The Role of Sphingosine-1 Phosphate and Ceramide-1 Phosphate in Trafficking of Normal Stem Cells and Cancer Cells. Expert. Opin. Ther. Targets 2014, 18, 95–107.
- Yamada, Y.; Minatoguchi, S.; Baba, S.; Shibata, S.; Takashima, S.; Wakao, S.; Okura, H.; Dezawa, M.; Minatoguchi, S. Human Muse Cells Reduce Myocardial Infarct Size and Improve Cardiac Function without Causing Arrythmias in a Swine Model of Acute Myocardial Infarction. PLoS ONE 2022, 17, e0265347.
- Kuroda, Y.; Wakao, S.; Kitada, M.; Murakami, T.; Nojima, M.; Dezawa, M. Isolation, Culture and Evaluation of Multilineage-Differentiating Stress-Enduring (Muse) Cells. Nat. Protoc. 2013, 8, 1391–1415.
- Gill, J.K.; Rehsia, S.K.; Verma, E.; Sareen, N.; Dhingra, S. Stem Cell Therapy for Cardiac Regeneration: Past, Present, and Future. Can. J. Physiol. Pharmacol. 2024, 102, 3.
- Yanamandala, M.; Zhu, W.; Garry, D.J.; Kamp, T.J.; Hare, J.M.; Jun, H.; Yoon, Y.; Bursac, N.; Prabhu, S.D.; Dorn, G.W.; et al. Overcoming the Roadblocks to Cardiac Cell Therapy Using Tissue Engineering. J. Am. Coll. Cardiol. 2017, 70, 766–775.
- Sato, Y.; Bando, H.; Piazza, M.D.; Gowing, G.; Herberts, C.; Jackman, S.; Leoni, G.; Libertini, S.; MacLachlan, T.; McBlane, J.W.; et al. Tumorigenicity Assessment of Cell Therapy Products: The Need for Global Consensus and Points to Consider. Cytotherapy 2019, 21, 1095–1111.
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med. Iran 2017, 55, 6–23.
- Johnston, P.V. Gary Gerstenblith Stem Cells for Cardiac Surgical Disease. In Johns Hopkins Textbook of Cardiothoracic Surgery; McGraw-Hill Companies New York: New York, NY, USA, 2014; p. 1443. ISBN 978-0-07-166350-2.
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone Marrow Cells Regenerate Infarcted Myocardium. Nature 2001, 410, 701–705.
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of Bone Marrow Mononuclear Cells Into Ischemic Myocardium Enhances Collateral Perfusion and Regional Function via Side Supply of Angioblasts, Angiogenic Ligands, and Cytokines. Circulation 2001, 104, 1046–1052.
- Bel, A.; Messas, E.; Agbulut, O.; Richard, P.; Samuel, J.-L.; Bruneval, P.; Hagège, A.A.; Menasché, P. Transplantation of Autologous Fresh Bone Marrow Into Infarcted Myocardium: A Word of Caution. Circulation 2003, 108, II-247–II-252.
- de Silva, R.; Raval, A.N.; Hadi, M.; Gildea, K.M.; Bonifacino, A.C.; Yu, Z.-X.; Yau, Y.Y.; Leitman, S.F.; Bacharach, S.L.; Donahue, R.E.; et al. Intracoronary Infusion of Autologous Mononuclear Cells from Bone Marrow or Granulocyte Colony-Stimulating Factor-Mobilized Apheresis Product May Not Improve Remodelling, Contractile Function, Perfusion, or Infarct Size in a Swine Model of Large Myocardial Infarction. Eur. Heart J. 2008, 29, 1772–1782.
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El, N.B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic Stem Cell Therapy in Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 2329–2338.
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A Critical Challenge: Dosage-Related Efficacy and Acute Complication Intracoronary Injection of Autologous Bone Marrow Mesenchymal Stem Cells in Acute Myocardial Infarction. Int. J. Cardiol. 2013, 168, 3191–3199.
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Ellis, S.G. One-Year Follow-up of Intracoronary Stem Cell Delivery on Left Ventricular Function following ST-Elevation Myocardial Infarction. JAMA 2014, 311, 301–302.
- Zhao, Q.; Sun, Y.; Xia, L.; Chen, A.; Wang, Z. Randomized Study of Mononuclear Bone Marrow Cell Transplantation in Patients with Coronary Surgery. Ann. Thorac. Surg. 2008, 86, 1833–1840.
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540.
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary Infusion of Wharton’s Jelly-Derived Mesenchymal Stem Cells in Acute Myocardial Infarction: Double-Blind, Randomized Controlled Trial. BMC Med. 2015, 13, 162.
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary Cardiosphere-Derived Cells for Heart Regeneration after Myocardial Infarction (CADUCEUS): A Prospective, Randomised Phase 1 Trial. Lancet 2012, 379, 895–904.
- Medscape Medical News: Harvard, Brigham Call for Retraction of 31 Papers by Disgraced Cardiac Stem Cell Doc. Available online: https://www.medscape.com/viewarticle/903475 (accessed on 26 February 2024).
- The BMJ News: NEJM Retracts Article from Former Researcher Once Hailed as Heart Stem Cell Pioneer. Available online: https://www.bmj.com/content/363/bmj.k4432 (accessed on 26 February 2024).
- Afzal, M.R.; Samanta, A.; Shah, Z.I.; Jeevanantham, V.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease. Circ. Res. 2015, 117, 558–575.
- Ali-Hasan-Al-Saegh, S.; Mirhosseini, S.J.; Lotfaliani, M.-R.; Dehghan, H.R.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Rezaeisadrabadi, M.; Ghaffari, N.; Vahabzadeh, V.; Jebran, A.F.; et al. Transplantation of Bone Marrow Stem Cells during Cardiac Surgery. Asian Cardiovasc. Thorac. Ann. 2015, 23, 363–374.
- Ala, M. The Beneficial Effects of Mesenchymal Stem Cells and Their Exosomes on Myocardial Infarction and Critical Considerations for Enhancing Their Efficacy. Aging Res. Rev. 2023, 89, 101980.
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal Stem Cell Injection after Myocardial Infarction Improves Myocardial Compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203.
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine Action Accounts for Marked Protection of Ischemic Heart by Akt-Modified Mesenchymal Stem Cells. Nat. Med. 2005, 11, 367–368.
- Haider, H.K.; Jiang, S.; Idris, N.M.; Ashraf, M. IGF-1-Overexpressing Mesenchymal Stem Cells Accelerate Bone Marrow Stem Cell Mobilization via Paracrine Activation of SDF-1alpha/CXCR4 Signaling to Promote Myocardial Repair. Circ. Res. 2008, 103, 1300–1308.
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M.; Minatoguchi, S. Stem Cell Therapy for Acute Myocardial Infarction—Focusing on the Comparison between Muse Cells and Mesenchymal Stem Cells. J. Cardiol. 2022, 80, 80–87.
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301.
- The SCIENCE Investigators. Effect of Allogeneic Adipose Tissue-Derived Mesenchymal Stromal Cell Treatment in Chronic Ischaemic Heart Failure with Reduced Ejection Fraction—The Science Trial. Eur. J. Heart Fail. 2023, 25, 576–587.
- Eisenberg, L.M.; Eisenberg, C.A. Adult Stem Cells and Their Cardiac Potential. Anat. Rec. Part. A Discov. Mol. Cell. Evol. Biol. 2004, 276A, 103–112.
- Mehanna, R.A.; Essawy, M.M.; Barkat, M.A.; Awaad, A.K.; Thabet, E.H.; Hamed, H.A.; Elkafrawy, H.; Khalil, N.A.; Sallam, A.; Kholief, M.A.; et al. Cardiac Stem Cells: Current Knowledge and Future Prospects. World J. Stem Cells 2022, 14, 1–40.
- Bolli, R.; Tang, X.-L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation 2013, 128, 122–131.
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Preclinical Studies of Stem Cell Therapy for Heart Disease. Circ. Res. 2018, 122, 1006–1020.
- Mahmud, S.; Alam, S.; Emon, N.U.; Boby, U.H.; Kamruzzaman; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Tahamina, A.; Rudra, S.; Ajrin, M. Opportunities and Challenges in Stem Cell Therapy in Cardiovascular Diseases: Position Standing in 2022. Saudi Pharm. J. 2022, 30, 1360–1371.
- Expression of Concern: The SCIPIO Trial. Lancet 2014, 383, 1279.
- Xiao, W.; Shi, J. Application of Adipose-Derived Stem Cells in Ischemic Heart Disease: Theory, Potency, and Advantage. Front. Cardiovasc. Med. 2024, 11, 1324447.
- Wang, J.; An, M.; Haubner, B.J.; Penninger, J.M. Cardiac Regeneration: Options for Repairing the Injured Heart. Front. Cardiovasc. Med. 2023, 9, 981982.
- Guo, Q.-Y.; Yang, J.-Q.; Feng, X.-X.; Zhou, Y.-J. Regeneration of the Heart: From Molecular Mechanisms to Clinical Therapeutics. Mil. Med. Res. 2023, 10, 18.
- Chen, K.; Huang, Y.; Singh, R.; Wang, Z.Z. Arrhythmogenic Risks of Stem Cell Replacement Therapy for Cardiovascular Diseases. J. Cell Physiol. 2020, 235, 6257–6267.
- Carbone, R.G.; Negrini, S.; Murdaca, G.; Fontana, V.; Puppo, F. Stem Cells Treatment in Chronic Ischemic Heart Disease: A Narrative Review. Am. J. Stem Cells 2023, 12, 65–72.
- Harland, N.; Knoll, J.; Amend, B.; Abruzzese, T.; Abele, H.; Jakubowski, P.; Stenzl, A.; Aicher, W.K. Xenogenic Application of Human Placenta-Derived Mesenchymal Stromal Cells in a Porcine Large Animal Model. Cell Transpl. 2024, 33, 9636897241226737.
- Liu, Y.-W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human Embryonic Stem Cell–Derived Cardiomyocytes Restore Function in Infarcted Hearts of Non-Human Primates. Nat. Biotechnol. 2018, 36, 597–605, Erratum in Nat. Biotechnol. 2018, 36, 899.
- Askar, S.F.A.; Ramkisoensing, A.A.; Atsma, D.E.; Schalij, M.J.; de Vries, A.A.F.; Pijnappels, D.A. Engraftment Patterns of Human Adult Mesenchymal Stem Cells Expose Electrotonic and Paracrine Proarrhythmic Mechanisms in Myocardial Cell Cultures. Circ. Arrhythmia Electrophysiol. 2013, 6, 380–391.
- Chandrasekhar, S.K.; Thankam, F.G.; Ouseph, J.C.; Agrawal, D.K. Myocardial Tissue Engineering: Fundamentals and Future. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–51. ISBN 978-0-12-824064-9.
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current Research Trends and Challenges in Tissue Engineering for Mending Broken Hearts. Life Sci. 2019, 229, 233–250.
- Lou, X.; Tang, Y.; Ye, L.; Pretorius, D.; Fast, V.G.; Kahn-Krell, A.M.; Zhang, J.; Zhang, J.; Qiao, A.; Qin, G.; et al. Cardiac Muscle Patches Containing Four Types of Cardiac Cells Derived from Human Pluripotent Stem Cells Improve Recovery from Cardiac Injury in Mice. Cardiovasc. Res. 2023, 119, 1062–1076.
- Masumoto, H.; Ikuno, T.; Takeda, M.; Fukushima, H.; Marui, A.; Katayama, S.; Shimizu, T.; Ikeda, T.; Okano, T.; Sakata, R.; et al. Human iPS Cell-Engineered Cardiac Tissue Sheets with Cardiomyocytes and Vascular Cells for Cardiac Regeneration. Sci. Rep. 2014, 4, 6716.
- Ishigami, M.; Masumoto, H.; Ikuno, T.; Aoki, T.; Kawatou, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K.; Minatoya, K. Human iPS Cell-Derived Cardiac Tissue Sheets for Functional Restoration of Infarcted Porcine Hearts. PLoS ONE 2018, 13, e0201650.
- Zhang, L.; Guo, J.; Zhang, P.; Xiong, Q.; Wu, S.C.; Xia, L.; Roy, S.S.; Tolar, J.; O’Connell, T.D.; Kyba, M.; et al. Derivation and High Engraftment of Patient-Specific Cardiomyocyte Sheet Using Induced Pluripotent Stem Cells Generated from Adult Cardiac Fibroblast. Circ. Heart Fail. 2015, 8, 156–166.
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem Cell-Based Approaches in Cardiac Tissue Engineering: Controlling the Microenvironment for Autologous Cells. Biomed. Pharmacother. 2021, 138, 111425.
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell–Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730.
- Xiang, Z.; Liao, R.; Kelly, M.S.; Spector, M. Collagen–GAG Scaffolds Grafted onto Myocardial Infarcts in a Rat Model: A Delivery Vehicle for Mesenchymal Stem Cells. Tissue Eng. 2006, 12, 2467–2478.
- Simpson, D.; Liu, H.; Fan, T.-H.M.; Nerem, R.; Dudley, S.C., Jr. A Tissue Engineering Approach to Progenitor Cell Delivery Results in Significant Cell Engraftment and Improved Myocardial Remodeling. Stem Cells 2007, 25, 2350–2357.
- Jin, J.; Jeong, S.I.; Shin, Y.M.; Lim, K.S.; Shin, H.S.; Lee, Y.M.; Koh, H.C.; Kim, K.-S. Transplantation of Mesenchymal Stem Cells within a Poly(Lactide-Co-ε-Caprolactone) Scaffold Improves Cardiac Function in a Rat Myocardial Infarction Model. Eur. J. Heart Fail. 2009, 11, 147–153.
- Zhang, J.; Li, J.; Qu, X.; Liu, Y.; Harada, A.; Hua, Y.; Yoshida, N.; Ishida, M.; Tabata, A.; Sun, L.; et al. Development of a Thick and Functional Human Adipose-Derived Stem Cell Tissue Sheet for Myocardial Infarction Repair in Rat Hearts. Stem Cell Res. Ther. 2023, 14, 380.
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered Mesenchymal Stem Cells Repair Scarred Myocardium after Myocardial Infarction. Nat. Med. 2006, 12, 459–465.
- Huang, C.-C.; Tsai, H.-W.; Lee, W.-Y.; Lin, W.-W.; Chen, D.-Y.; Hung, Y.-W.; Chen, J.-W.; Hwang, S.-M.; Chang, Y.; Sung, H.-W. A Translational Approach in Using Cell Sheet Fragments of Autologous Bone Marrow-Derived Mesenchymal Stem Cells for Cellular Cardiomyoplasty in a Porcine Model. Biomaterials 2013, 34, 4582–4591.
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749.
- Mei, X.; Cheng, K. Recent Development in Therapeutic Cardiac Patches. Front. Cardiovasc. Med. 2020, 7, 610364.
