Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The advent of PET/MRI, which combines metabolic PET information with high anatomical detail from MRI, has emerged as a promising tool for breast cancer diagnosis, staging, treatment response assessment, and restaging. Technical advancements including the integration of PET and MRI, considerations in patient preparation, and optimized imaging protocols contribute to the success of dedicated breast and whole-body PET/MRI.
- breast cancer
- PET/MRI
- 8F-FDG PET/CT
1. Introduction
Breast cancer is the cancer with the highest incidence and mortality among women worldwide [1]. Early detection and precise staging are crucial for effective treatment and improved patient outcomes [2]. Mammography, ultrasound (US), and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) are well-established local-regional imaging methods for breast cancer. Bone scintigraphy and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) are also available to evaluate distant metastasis [3].
18F-FDG PET/CT has been widely validated for various cancers, playing an important role in diagnosis, initial staging, therapy response evaluation, and restaging [4]. However, its application for breast cancer in terms of detection, differential diagnosis of benign from malignant lesions, and local tumor staging is not recommended. This is due to its high false-negative rate for small (<1 cm) and low-grade breast cancer, a high false-positive rate for local benign breast disease, and low sensitivity for the detection of axillary lymph node metastasis [5]. Consequently, researchers have explored various novel PET radiotracers targeting molecular factors. Examples include 18F-fluoroestradiol (FES) for estrogen receptor (ER), 89Zr-Df-trastuzumab for human epidermal growth factor receptor-2 (HER2), 68Ga-fibroblast-activation-protein-inhibitor (FAPI) for fibroblast activation protein (FAP), and 18F-fluoromisonidazole (FMISO) for hypoxia. In May 2022, the United States Food and Drug Administration approved 18F-FES for patients with recurrent and metastatic breast cancer, serving as an adjunct to biopsy for detecting ER-positive lesions [3].
PET/MRI, which combines the metabolic information of PET with the high anatomical details of MRI, has been suggested as a promising synergistic imaging modality for cancer. Since MRI is highly sensitive for breast cancer and does not expose the patient to radiation, PET/MRI is under active investigation across the spectrum of diagnosis, staging, treatment response assessment, and restaging of breast cancer [6].
2. 18F-FDG PET/MRI for Breast Cancer
2.1. Diagnosis
Breast MRI is the most sensitive modality for detecting breast cancer [7]. However, the moderately specific nature of breast MRI results in false-positive findings, necessitating additional imaging and biopsy [8]. Thus, reducing false-positive findings from breast MRI has significant potential impact by avoiding additional biopsies, reducing cost, decreasing patient anxiety, and minimizing time to surgery.
18F-FDG PET has a limited sensitivity in small breast lesion with both false-negative and false-positive findings as a benign lesion can have an increased uptake. However, the addition of 18F-FDG PET with multiparametric MRI has been suggested to increase the specificity, especially when the size of breast lesions is more than 10 mm [9]. Furthermore, the addition of dedicated prone breast PET/MRI to supine whole-body imaging is reported to be more sensitive than whole-body-only supine imaging. In a study involving 38 prospectively enrolled patients with 56 breast cancer lesions, dedicated prone breast 18F-FDG PET/MRI, combined with supine whole-body imaging, correctly identified breast cancers in 97% of cases (37/38). In contrast, supine whole-body-only imaging missed five patients (87%, 33/38) [10]. The unidentified patient in dedicated prone breast 18F-FDG PET/MRI had a pT1a tumor measuring 5 mm without radiotracer uptake and considered a benign lesion. The missed five patients in supine whole-body-only 18F-FDG PET/MRI had small lesions ranging from 5 to 13 mm.
With recent advances in artificial intelligence (AI) and its applications in medical imaging, Romeo et al. demonstrated that the AI-based radiomics model, with features extracted from simultaneous multiparametric 18F-FDG PET/MRI, achieved high accuracy in discriminating between benign and malignant breast lesions, with an AUC of 0.983 [11]. Its sensitivity was not statistically different from the clinical interpretation by experts (100% for AI-based radiomics model vs. 95.3% for clinical interpretation), but specificity was higher for the AI-based radiomics model (94.3% vs. 73.7%, respectively), indicating the potential to decrease false-positive findings in benign breast lesions.
While 18F-FDG PET/MRI is not currently recommended for breast cancer diagnosis, its utilization could improve the diagnostic accuracy of MRI and potentially enable a less invasive, comprehensive diagnostic strategy.
2.2. Initial Staging
The American Joint Committee on Cancer (AJCC) anatomic TNM staging system for breast cancer includes the extent of the tumor (T), the spread to regional lymph nodes (N), and distant metastasis (M). It emphasizes that comprehensive initial anatomic staging using mammography, US, and MRI is crucial for guiding patient treatment decisions [12].
MRI has demonstrated greater accuracy than conventional imaging methods in assessing the extent of breast tumors [13]. Consequently, 18F-FDG PET/MRI is proposed to be superior for T-staging compared to conventional mammography, ultrasound (US), and 18F-FDG PET/CT, and is, at the very least, equivalent to breast MRI alone. In a study by Grueneisen et al., involving 49 patients with 83 biopsy-proven invasive breast cancers, mostly at more than T1c stage, no significant difference was observed in correct T-staging between 18F-FDG PET/MRI and MRI. However, both modalities were significantly more accurate than 18F-FDG PET/CT (PET/MRI and MRI, 82%; PET/CT, 68%) [14].
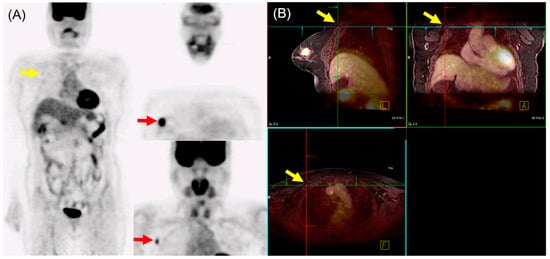
In N staging, early studies initially reported the performance of 18F-FDG PET/MRI as either equivalent to or inferior to MRI alone [14][15]. However, Morawitz et al. demonstrated the diagnostic superiority of 18F-FDG PET/MRI over MRI and CT in determining the regional lymph node status. Their prospective double-center study involved 182 patients with newly diagnosed, treatment-naïve breast cancer [16]. 18F-FDG PET/MRI detected significantly more nodal-positive patients than MRI and CT. Moreover, across all lymph node stations (axillary, supraclavicular, and internal mammary stations), 18F-FDG PET/MRI identified significantly more lymph node metastases compared to MRI and CT. Consequently, 18F-FDG PET/MRI resulted in nodal upstaging in 30 patients compared to MRI and in 41 patients compared to CT. No downstaging occurred in 18F-FDG PET/MRI compared to MRI or CT. MRI upstaged nodes in 15 patients and downstaged nodes in 6 patients compared to CT. Additionally, simple imaging features from 18F-FDG PET/MRI and MRI can be utilized for N staging in patients with newly diagnosed breast cancer through machine-learning–based prediction models, exhibiting high accuracy [17]. The diagnostic accuracy for MRI features was 87.5% for both the machine-learning algorithm and radiologists. For 18F-FDG PET/MRI, the diagnostic accuracy was 91.2% and 89.3% for the machine-learning algorithm and radiologists, respectively, with no significant difference. An example of 18F-FDG PET/MRI for initial N staging in a patient with breast cancer is shown in Figure 1.

Figure 1. 18F-FDG PET/MRI for initial N staging in a patient with breast cancer. (A) PET images revealed hypermetabolic right breast cancer with right axillary lymph node metastasis (red arrow). Additionally, inconclusive mild hypermetabolic uptake was found in the right interpectoral area (yellow arrow). (B) PET/MRI identified right interpectoral lymph node metastasis with gadolinium-contrast enhancement on T1-w MRI (yellow arrow).
Currently, ongoing clinical trials are evaluating the axillary staging performance of 18F-FDG PET/MRI compared to sentinel node biopsy (SNB) in both advanced and early breast cancers [18]. These trials consist of two prospective comparative single-center studies conducted in different settings. In the first trial (ClinicalTrials.gov Identifier: NCT04826211), the staging performance of 18F-FDG PET/MRI is compared with SNB in patients with breast cancer undergoing primary systemic therapy (PST) for positive axillary lymph nodes at diagnosis. Initial staging and post-PST 18F-FDG PET/MRI results, which are used to plan surgery, will be compared with the results of SNB. In the second trial (ClinicalTrials.gov Identifier: NCT04829643), the axillary staging of 18F-FDG PET/MRI is compared with SNB in patients with early breast cancer undergoing surgery. The results from these trials have the potential to offer patients a less invasive and de-escalated axillary surgery with improved outcomes.
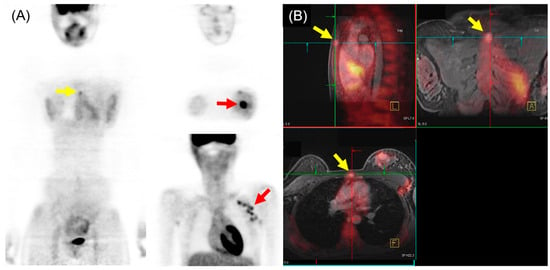
In the comparison of 18F-FDG PET/MRI and 18F-FDG PET/CT for M staging of breast cancer, higher sensitivity and lower specificity of 18F-FDG PET/MRI were generally found, particularly for osseous and/or hepatic metastases [19]. Recent meta-analysis, including 16 articles involving 1261 patients, indicated that 18F-FDG PET/MRI showed superior sensitivity and similar specificity to 18F-FDG PET/CT in detecting bone metastases in patients with breast cancer [20]. Another meta-analysis with a subgroup using three studies (182 patients) reported that 18F-FDG PET/MRI had higher sensitivity and specificity for detecting distant metastasis of breast cancer than 18F-FDG PET/CT [21]. The pooled sensitivity, specificity, and AUC for 18F-FDG PET/MRI were 95%, 96%, and 0.98, respectively. For 18F-FDG PET/CT, the pooled sensitivity, specificity, and AUC were 87%, 94%, and 0.94, respectively. An example of 18F-FDG PET/MRI for initial M staging in a patient with breast cancer is shown in Figure 2.

Figure 2. 18F-FDG PET/MRI for initial M staging in a patient with breast cancer. (A) PET images revealed hypermetabolic left breast cancer with left axillary lymph node metastases (red arrow). Additionally, inconclusive mild hypermetabolic uptake was observed in the sternum area (yellow arrow). (B) PET/MRI identified sternum metastasis with gadolinium-contrast enhancement on T1-w MRI (yellow arrow).
2.3. Therapy Response Assessment
While the assessment of tumor response after therapy still relies on changes in size, 18F-FDG PET/MRI shows greater potential than conventional anatomical imaging. 18F-FDG PET/MRI can provide functional data such as metabolism (PET), cell proliferation (DWI), and neoangiogenesis (DCE-MRI), along with anatomical details, in the assessment and early prediction of systemic therapy response.
An early study with nine patients by Jena et al. reported that PET metabolic parameters, such as maximum standardized uptake value (SUVmax), and DCE-MRI pharmacokinetic parameters, such as Ktrans (volume transfer constant between blood plasma and interstitial space), obtained from simultaneous 18F-FDG PET/MRI were reduced after chemotherapy in patients who have responded to therapy [22]. Wang et al. suggested that changes in combined PET and MRI parameters, such as SUVmax, total lesion glycolysis (TLG), and minimum ADC (ADCmin), in sequential 18F-FDG PET/MRIs obtained before and after the first or second cycle of neoadjuvant chemotherapy (NCT), predict treatment response more accurately than individual PET/MRI parameters [23]. Cho et al. demonstrated that TLG obtained from PET and signal enhancement ratio (SER) from MRI in 18F-FDG PET/MRI predicted non-pathological complete response (pCR) after the first cycle of NCT in patients with breast cancer [24]. Sekine et al. evaluated the performance of 18F-FDG PET/MRI, mammography, and US in 74 patients with breast cancer in prediction of pCR after NCT [25]. The prediction of pCR by 18F-FDG PET/MRI depended on the absence of detectable enhancement on MRI and/or lack of meaningful uptake on PET. The overall sensitivity of 18F-FDG PET/MRI, mammography, and US were 72%, 71%, and 17%, respectively. The overall specificity of 18F-FDG PET/MRI, mammography, and US were 79%, 80%, and 91%, respectively. In another study, de Mooij et al. conducted a prospective study involving 41 patients with 42 primary invasive breast cancers who underwent NCT before surgery [26]. Qualitative evaluation using 18F-FDG PET/MRI after NCT predicted the therapy response in the primary tumor but not the response in axillary lymph node metastasis. When compared to qualitative evaluation after NCT, combining quantitative variables such as decreased SUVmax and SER in the primary tumor and axillary lymph node metastasis from sequential 18F-FDG PET/MRI during NCT improved the evaluation and prediction of response to NCT. Moreover, deep learning techniques and radiomics-based analysis may be helpful in predicting the response to NCT using 18F-FDG PET/MRI [27][28].
2.4. Restaging
Early detection and characterization of local-regional recurrence and distant metastasis are essential for optimal treatment and prognosis in patients with previously treated breast cancer. Curative surgery or radiation therapy may be available for local-regional recurrence, whereas palliative systemic therapy is necessary for distant metastases [29].
18F-FDG PET/CT has been recommended as a helpful imaging modality in situations where standard staging studies are equivocal or suspicious [5]. In a prospective study by Sawiki et al., 21 patients with 134 suspected breast cancer recurrent lesions underwent whole-body PET/CT with iodinated contrast. Subsequently, they underwent PET/MRI with a gadolinium-based contrast agent. PET/CT and PET/MRI were performed in a single injection of 18F-FDG [30]. For patient-based analysis, 18F-FDG PET/MRI, 18F-FDG PET/CT, and MRIPET/MRI correctly identified all 17 patients with cancer recurrence. CTPET/CT identified 15 of the 17 patients correctly (88.2%). In lesion-based analysis, 18F-FDG PET/MRI, 18F-FDG PET/CT, MRIPET/MRI, and CTPET/CT correctly identified 98.5% (132/134), 94.8% (127/134), 88.1% (118/134), and 57.5% (77/134) of all lesions, respectively. Notably, bone, lymph node (<10 mm), and liver metastases were missed by images, particularly by CTPET/CT. Interobserver agreement was substantial for both 18F-FDG PET/MRI and 18F-FDG PET/CT, moderate for MRIPET/MRI, and fair for CTPET/CT.
3. Conclusions
PET/MRI is a multimodal imaging technique that integrates metabolic and anatomical information. Recent technical advancements have enabled the simultaneous acquisition of PET and MRI, enhancing diagnostic accuracy. Consequently, 18F-FDG PET/MRI has demonstrated potential in the diagnosis, initial staging, therapy response assessment, and restaging of patients with breast cancer. The ongoing exploration of novel targets for PET radiotracers beyond glucose metabolism shows promise for more targeted approaches to breast cancer diagnosis and treatment. Further investigations and a better understanding are necessary to define the role and optimal patient population for PET/MRI and novel radiotracers in breast cancer.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines12010172
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Roginski, M.; Sifaki-Pistolla, D.; Stomby, A.; Velivasaki, G.; Faresjö, T.; Lionis, C.; Faresjö, Å. Paradoxes of breast cancer incidence and mortality in two corners of Europe. BMC Cancer 2022, 22, 1123.
- Fowler, A.M.; Strigel, R.M. Clinical advances in PET-MRI for breast cancer. Lancet Oncol. 2022, 23, e32–e43.
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354.
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722.
- Pujara, A.C.; Kim, E.; Axelrod, D.; Melsaether, A.N. PET/MRI in Breast Cancer. J. Magn. Reson. Imaging 2019, 49, 328–342.
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536.
- Chen, H.L.; Zhou, J.Q.; Chen, Q.; Deng, Y.C. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤2 cm) breast cancer. Medicine 2021, 100, e26531.
- Bitencourt, A.G.; Lima, E.N.; Chojniak, R.; Marques, E.F.; Souza, J.A.; Andrade, W.P.; Guimarães, M.D. Can 18F-FDG PET improve the evaluation of suspicious breast lesions on MRI? Eur. J. Radiol. 2014, 83, 1381–1386.
- Kirchner, J.; Grueneisen, J.; Martin, O.; Oehmigen, M.; Quick, H.H.; Bittner, A.K.; Hoffmann, O.; Ingenwerth, M.; Catalano, O.A.; Heusch, P.; et al. Local and whole-body staging in patients with primary breast cancer: A comparison of one-step to two-step staging utilizing 18F-FDG-PET/MRI. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2328–2337.
- Romeo, V.; Clauser, P.; Rasul, S.; Kapetas, P.; Gibbs, P.; Baltzer, P.A.T.; Hacker, M.; Woitek, R.; Helbich, T.H.; Pinker, K. AI-enhanced simultaneous multiparametric 18F-FDG PET/MRI for accurate breast cancer diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 596–608.
- Teichgraeber, D.C.; Guirguis, M.S.; Whitman, G.J. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. AJR Am. J. Roentgenol. 2021, 217, 278–290.
- Panico, C.; Ferrara, F.; Woitek, R.; D’Angelo, A.; Di Paola, V.; Bufi, E.; Conti, M.; Palma, S.; Cicero, S.L.; Cimino, G.; et al. Staging Breast Cancer with MRI, the T. A Key Role in the Neoadjuvant Setting. Cancers 2022, 14, 5786.
- Grueneisen, J.; Nagarajah, J.; Buchbender, C.; Hoffmann, O.; Schaarschmidt, B.M.; Poeppel, T.; Forsting, M.; Quick, H.H.; Umutlu, L.; Kinner, S. Positron Emission Tomography/Magnetic Resonance Imaging for Local Tumor Staging in Patients with Primary Breast Cancer: A Comparison with Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging. Investig. Radiol. 2015, 50, 505–513.
- Botsikas, D.; Kalovidouri, A.; Becker, M.; Copercini, M.; Djema, D.A.; Bodmer, A.; Monnier, S.; Becker, C.D.; Montet, X.; Delattre, B.M.; et al. Clinical utility of 18F-FDG-PET/MR for preoperative breast cancer staging. Eur. Radiol. 2016, 26, 2297–2307.
- Morawitz, J.; Bruckmann, N.M.; Dietzel, F.; Ullrich, T.; Bittner, A.K.; Hoffmann, O.; Ruckhäberle, E.; Mohrmann, S.; Häberle, L.; Ingenwerth, M.; et al. Comparison of nodal staging between CT, MRI, and -FDG PET/MRI in patients with newly diagnosed breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 992–1001.
- Morawitz, J.; Sigl, B.; Rubbert, C.; Bruckmann, N.M.; Dietzel, F.; Häberle, L.J.; Ting, S.; Mohrmann, S.; Ruckhäberle, E.; Bittner, A.K.; et al. Clinical Decision Support for Axillary Lymph Node Staging in Newly Diagnosed Breast Cancer Patients Based on 18F-FDG PET/MRI and Machine Learning. J. Nucl. Med. 2023, 64, 304–311.
- Di Micco, R.; Santurro, L.; Gasparri, M.L.; Zuber, V.; Cisternino, G.; Baleri, S.; Morgante, M.; Rotmensz, N.; Canevari, C.; Gallivanone, F.; et al. PET/MRI for Staging the Axilla in Breast Cancer: Current Evidence and the Rationale for SNB vs. PET/MRI Trials. Cancers 2021, 13, 3571.
- de Mooij, C.M.; Sunen, I.; Mitea, C.; Lalji, U.C.; Vanwetswinkel, S.; Smidt, M.L.; van Nijnatten, T.J.A. Diagnostic performance of PET/computed tomography versus PET/MRI and diffusion-weighted imaging in the N- and M-staging of breast cancer patients. Nucl. Med. Commun. 2020, 41, 995–1004.
- Xia, L.; Lai, J.; Huang, D.; Qiu, S.; Hu, H.; Luo, Y.; Cao, J. Comparing the diagnostic efficacy of FDG PET/CT and FDG PET/MRI for detecting bone metastases in breast cancer: A meta-analysis. Radiol Oncol. 2023, 57, 299–309.
- Zhang, C.; Liang, Z.; Liu, W.; Zeng, X.; Mo, Y. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: A meta-analysis. BMC Cancer 2023, 23, 37.
- Jena, A.; Taneja, S.; Singh, A.; Negi, P.; Mehta, S.B.; Ahuja, A.; Singhal, M.; Sarin, R. Association of pharmacokinetic and metabolic parameters derived using simultaneous PET/MRI: Initial findings and impact on response evaluation in breast cancer. Eur. J. Radiol. 2017, 92, 30–36.
- Wang, J.; Shih, T.T.; Yen, R.F. Multiparametric Evaluation of Treatment Response to Neoadjuvant Chemotherapy in Breast Cancer Using Integrated PET/MR. Clin. Nucl. Med. 2017, 42, 506–513.
- Cho, N.; Im, S.A.; Cheon, G.J.; Park, I.A.; Lee, K.H.; Kim, T.Y.; Kim, Y.S.; Kwon, B.R.; Lee, J.M.; Suh, H.Y.; et al. Integrated 18F-FDG PET/MRI in breast cancer: Early prediction of response to neoadjuvant chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 328–339.
- Sekine, C.; Uchiyama, N.; Watase, C.; Murata, T.; Shiino, S.; Jimbo, K.; Iwamoto, E.; Takayama, S.; Kurihara, H.; Satomi, K.; et al. Preliminary experiences of PET/MRI in predicting complete response in patients with breast cancer treated with neoadjuvant chemotherapy. Mol. Clin. Oncol. 2022, 16, 50.
- de Mooij, C.M.; van Nijnatten, T.J.A.; Goorts, B.; Kooreman, L.F.S.; Raymakers, I.W.M.; van Meijl, S.P.L.; de Boer, M.; Keymeulen, K.B.M.I.; Wildberger, J.E.; Mottaghy, F.M.; et al. Prediction of Primary Tumour and Axillary Lymph Node Response to Neoadjuvant Chemo(Targeted) Therapy with Dedicated Breast FDG PET/MRI in Breast Cancer. Cancers 2023, 15, 401.
- Umutlu, L.; Kirchner, J.; Bruckmann, N.M.; Morawitz, J.; Antoch, G.; Ting, S.; Bittner, A.K.; Hoffmann, O.; Häberle, L.; Ruckhäberle, E.; et al. Multiparametric 18F-FDG PET/MRI-Based Radiomics for Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2022, 14, 1727.
- Choi, J.H.; Kim, H.A.; Kim, W.; Lim, I.; Lee, I.; Byun, B.H.; Noh, W.C.; Seong, M.K.; Lee, S.S.; Kim, B.I.; et al. Early prediction of neoadjuvant chemotherapy response for advanced breast cancer using PET/MRI image deep learning. Sci. Rep. 2020, 10, 21149.
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.L.; Hindié, E.; Mankoff, D. 18F-FDG PET/CT for Staging and Restaging of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 17S–26S.
- Sawicki, L.M.; Grueneisen, J.; Schaarschmidt, B.M.; Buchbender, C.; Nagarajah, J.; Umutlu, L.; Antoch, G.; Kinner, S. Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur. J. Radiol. 2016, 85, 459–465.
This entry is offline, you can click here to edit this entry!
