Terpenes and their derivatives comprise a diverse group of natural compounds with versatile medicinal properties. This article elucidates the general characteristics of fungal terpenes and terpenoids, encompassing their structure and biogenesis. The focal point of this work involves a comprehensive overview of these compounds, highlighting their therapeutic properties, mechanisms of action, and potential applications in treating specific skin conditions. Numerous isolated terpenes and terpenoids have demonstrated noteworthy anti-inflammatory and anti-microbial effects, rivalling or surpassing the efficacy of currently employed treatments for inflammation or skin infections. Due to their well-documented antioxidant and anti-cancer attributes, these compounds exhibit promise in both preventing and treating skin cancer. Terpenes and terpenoids sourced from fungi display the capability to inhibit tyrosinase, suggesting potential applications in addressing skin pigmentation disorders and cancers linked to melanogenesis dysfunctions.
1. Introduction
Fungi represent a valuable reservoir of bioactive molecules, serving as secondary metabolites with promising therapeutic attributes [
1]. These molecules encompass various compounds, including terpenes and terpenoids, which are distinguished by their diverse chemical structures [
2]. The heterogeneity within terpene compound classes contributes to a wide array of biological activities. From the well-established anti-cancer and anti-inflammatory properties of sesquiterpenes to the anti-microbial activity of monoterpenes, the chemical diversity exhibited by fungal terpenes and terpenoids provides a versatile toolkit for potential therapeutic applications [
3].
The skin, being the largest human organ, plays a pivotal role as a barrier against ex-ternal environmental factors and potential pathogens [
4]. Conventional treatments often come with limitations such as side effects and incomplete efficacy. In response to these challenges, there has been a heightened search for alternative and complementary therapeutic agents, with a growing focus on natural compounds [
5].
Fungi, acknowledged as a valuable source of bioactive compounds, have been integral to traditional medicine for centuries [
6]. Advances in analytical techniques and pharmacological studies have revealed the presence of numerous terpenes in various fungal species [
7]. Terpenes and terpenoids, distinguished by their unique chemical structures and diverse biological activities, have shown promise in modulating cellular processes, including anti-inflammatory, anti-oxidant, and immunomodulatory effects [
8,
9]. Moreover, these compounds have exhibited anti-oxidant properties, offering potential protection against oxidative stress—a prevalent factor in skin aging and certain dermatoses [
10]. These properties make fungal terpenoids appealing candidates for developing therapeutic interventions in dermatology [
11]. Understanding potential mechanisms of action is pivotal for recognizing the therapeutic significance of fungal terpenoids in dermatology. These compounds possess the ability to modulate cell signaling pathways, influence gene expression, and interact with key molecular targets involved in skin homeostasis. The specific mechanisms governing the impact of fungal terpenes and their derivatives on inflammatory responses, wound healing, and other dermatological processes are active areas of research that could contribute to the development of targeted therapies [
12].
2. General Characteristics and Occurrence of Terpenes and Terpenoids
Terpenoids, also referred to as isoprenoids, are organic chemical compounds produced by many plants, fungi, animals, bacteria, and other microbes [
13]. Coined by Dumas in 1866, the term “terpene” originates from the Latin word “turpentine” (
Balsamum terebinthinae) [
14]. In nature, the term “terpene” is employed to characterize compounds composed of isoprene units [
15]. The varied structures and functions of terpenes and their derivatives position them as subjects of research for commercial applications. These compounds have been substantiated to possess anti-cancer, anti-microbial, anti-fungal, anti-parasitic, anti-viral, anti-allergic, anti-spasmodic, anti-hyperglycemic, anti-inflammatory, and immunomodulatory effects. Additionally, they serve as natural insecticides and find utility as protective agents in the storage of agricultural products [
16]. Terpenes and terpenoids have applications in dermatology and the cosmetic industry as components in skincare products owing to their diverse therapeutic actions. Their antioxidant properties contribute to safeguarding skin cells from aging and UV-induced damage, while also hindering melanogenesis. Compounds such as retinoids, vitamin A metabolites, and various forms of vitamin E can counteract photoaging by modulating epidermal keratinization, inhibiting UV-induced matrix metalloproteinases, and suppressing pigmentation through the inhibition of the tyrosinase enzyme in melanin synthesis. Orally administered carotenoids, vitamin E, and other terpenoids like carnosic acid, squalene, and coenzyme Q10 are utilized in functional foods and supplements to enhance skin condition and overall health benefits [
17]. Terpenoids extracted from fungal species have exhibited anti-inflammatory effects by reducing nitric oxide (NO) and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. Additionally, they demonstrate anti-microbial effects against pathogens like
Escherichia coli,
Staphylococcus aureus, and
Bacillus cereus [
18,
19,
20]. With their advantageous properties, terpenes and their derivatives hold promise for the treatment of various skin conditions, prompting further research to enhance understanding and practical applications in dermatological therapy.
3. Biogenesis
Biogenetically, terpenes originate from isopentenyl diphosphate or its isomer dimethylallyldiphosphate [
21]. In 1887, chemist Otto Wallach first proposed the hypothesis that terpenes result from the polymerization of pentene (C
5H
8), also known as isoprene. The biosynthesis of terpenes begins with three acetyl-CoA units that generate mevalonic acid, serving as the precursor for isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) [
14]. Haemiterpenes, the simplest compounds in the terpene group, directly originate from IPPs, DMAPPs, or one of the intermediates in their biosynthetic pathway, such as mevalonic acid [
22]. The synthesis of monoterpenes initiates with the initial compound geranyl diphosphate (GPP), which forms through the phosphorylation of mevalonic acid [
15]. GPP can combine with IPP to produce farnesyl diphosphate (FPP), the initial compound for sesquiterpene synthesis. In the next step, FPP can condense with the IPP molecule to form geranyl-geranyl-PP (GGPP), a diterpene. The dimerization of two GGPPs then yields 16-trans-phytoene, a tetraterpene, from which carotenoids are synthesized. Condensation of two FPP molecules generates squalene, a precursor of triterpenoids, crucial to the synthesis of triterpenes and sterols [
15,
22].
All fungal terpenoid products originate from IPPs and DMAPPs, synthesized through the mevalonate pathway [
23]. The formation of individual compounds primarily occurs through the transformation of the starting compounds via processes such as cyclization, oxidation, attachment, or de-substitution [
21]. The significant diversity of terpenoids arises from the existence of numerous distinct terpene synthases, with some of them generating multiple by-products [
14].
4. Structure and Chemical Classification of Terpenes and Terpenoids
In 1953, Leopold Ružička formulated the isoprene principle, asserting that the carbon skeleton of terpenes consists of isoprene units linked by regular (head-tail) and irregular arrangements [
24]. Terpenes can be classified into different classes based on the number of isoprene units (
n) in the molecule: hemiterpenes (C
5H
8), monoterpenes (C
10H
16), sesquiterpenes (C
15H
24), diterpenes (C
20H
32), triterpenes (C
30H
48), tetraterpenes (C
40H
64), and polyterpenes (C
5H
8)
n. It is essential to note that terpenoids differ from terpenes; terpenes are simple, unsaturated hydrocarbons polymerized by isoprene units, while terpenoids belong to terpene derivatives with attached elements or functional groups, such as oxidized and nitrogenated branches, as well as methyl oxidized groups that can be removed and moved at different positions [
25,
26].
5. Pharmacological Activities in Skin Disorders
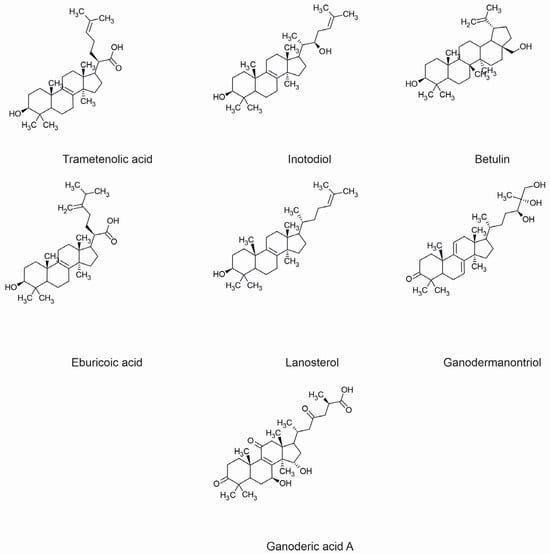
Many pharmacological activities exhibited by fungal terpenes and their derivatives have been proven. Several of them demonstrate significant properties for the treatment of dermatological diseases. The individual pharmacological properties of specific fungal terpenes and terpenoids are discussed below, along with their mechanisms of action and potential use in treating skin diseases. The chemical structures of selected compounds with applications in the prevention and treatment of skin diseases are shown in Figure 1.
Figure 1. Chemical structures of selected fungal terpenoids with applications in the prevention and treatment of skin diseases.
This entry is adapted from the peer-reviewed paper 10.3390/molecules29051183