2. Phytochemicals
2.1. Classification of Compounds
Phytochemicals (secondary metabolites) can be described as a class of compounds extracted from plants that display bioactive properties without being nutrients. They have numerous effects such as antioxidant, anti-inflammatory and anticancer. These effects depend on the concentration of each phytochemical in tissues, lumens or plasma. Comprising an enormous list of variable biochemical structures and characteristics, classification of these substances in groups is essential for further study. Broad categories are alkaloids, phenolics, organosulfur and terpenoids [
59]. Further subdivision based on biogenesis or structure is possible. This results in the following classes of phytochemicals: (a) Polyphenols that include flavones, isoflavones, flavonols, anthocyanins, lignans, phenols, stilbenes, tannins and coumarins; (b) carotenoids such as beta-carotene, lutein, cryptoxanthin and zeaxanthin; (c) glucosinolates; (d) polysaccharides including cellulose, pectins, gums, arabinoxylans, oligosaccharide, inulin and oligofructans; (e) lectins; (f) terpenes; (g) betalains; (h) capsaicinoids with the main one being capsaicin; (i) polyacetylenes; (j) allium compounds mainly methiin, propiin and isoalliin; (k) alkaloids that include well-known substances such as caffeine, morphine, nicotine, irinotecan, berberine, oxycodone, papaverine and (l) chlorophyll [
7,
60].
2.2. Phytochemical Administration and Delivery in IBD Patients
The use of phytochemicals in the form of herbal preparations, plant extracts or supplements, if effective, is of great value. The study of an effective extract can guide the research of its constituent phytochemicals to isolate the more potent ones [
13,
14]. In a similar way, inclusion of specific vegetables, legumes, fruits or grains as part of dietary consumption is a useful way to deliver anti-inflammatory and antioxidant phytochemicals to patients. Bioavailability in this mode of administration can be low. However, beneficiary effects have been noted for IBD patients following specific diets such as CDED, low-FODMAP and others [
61,
62,
63]. A confounding factor is that these diets also lower processed food intake, an independent variable of improved gut homeostasis. Also, synergistic boost of phytochemical action, e.g., by co-administration of probiotics and prebiotics, has been proposed for many diseases. Synbiotics, as some authors have been calling them, are under active research [
64]. Additionally, UC and CD have a number of pathogenetic and clinical differences that indicate their research as separate entities regarding phytochemical use. It is understandable that substances with proven antioxidant, anti-inflammatory or antibacterial effects in vitro or in animal IBD model studies would progress through preclinical trials to reach the stage of validation by RCT.
3. IBD
3.1. Pathophysiological Pathways of Inflammation and Oxidative Stress
Genetic factors appear to play an important role in IBD pathogenesis. Multiple genes are reported to be associated with increased risk for IBD. However, aside from the genetic factors, gut microbiota imbalance, environmental factors and immunological abnormalities are implicated in the pathogenesis of IBD [
3].
Gut microbiota seems to have an important role in the initiating events leading to IBD, as illustrated in the difference of gut microbiota composition between healthy study participants and IBD patients [
65]. Although genetic background is considered a major part in the pathogenesis of IBD, recent studies in twins, with one healthy twin and the other an IBD patient, demonstrate the importance of environmental factors on the onset of IBD. Specifically, the differences in gut microbiota between the twins seem to explain why one twin suffers from IBD whereas the other does not [
66]. The use of antibiotics leads to changes in the composition of gut microbiota and is also described as an initial part in the pathogenesis of IBD [
67]. Furthermore, changes in diet may induce inflammatory responses leading to IBD onset [
66].
Studies have further analyzed these gut microbiota imbalances, known as dysbiosis.
Clostridium spp. was significantly increased in IBD patients, whereas
Roseburia spp. and
Phascolarctobacterium spp., though abundant in healthy individuals, remained significantly low in IBD patients [
68].
Roseburia enhances the production of regulatory T-cells, thus expressing an anti-inflammatory effect in the intestinal tract [
69].
Phascolarctobacterium produces propionic acid, a short-chain fatty acid with known anti-inflammatory effects [
70]. The significantly lower concentration of both bacteria in the gut microbiota of IBD patients may contribute to the disease’s etiology. Furthermore,
Clostridium leptum group, a major bacterial group of gut microbiota, shows differences between healthy individuals and IBD patients in remission.
Faecalibacterium prausnitzii, a dominating bacteria of
Clostridium leptum group, show lower concentration in gut microbiota of IBD patients with both active disease and disease in remission [
71,
72]. Moreover, low concentration of
F. prausnitzii in the ileum of surgically treated patients with IBD is a risk factor for endoscopic recurrence. As a result,
F. prausnitzii has shown promise when administered as a probiotic. It appears to protect IBD patients from relapse due to the anti-inflammatory effects that have been reported both in vitro and in vivo [
73].
Recent articles on IBD epidemiology show a significant increase of CD’s prevalence in the developed world, as a result of progressive industrialization [
74]. This fact underlines the crucial role that environmental factors play in IBD pathogenesis. Diet remains a major risk factor. High intake of fruits and vegetables is associated with a reduced risk of CD [
75], whereas high intake of fatty and sugar-rich foods is associated with an increased risk of CD [
76]. Moreover, artificial food additives seem to worsen intestinal inflammation as they interfere with the function of the gut barrier [
75]. Another study shows that diets that contain high levels of animal protein induce a proinflammatory macrophage response [
77]. Other environmental factors, implicated in the pathogenesis of IBD, are appendectomy, medications or other iatrogenic factors, psychological stress and smoking [
78]. Although smoking worsens CD, it seems to produce a protective effect for UC, mainly due to the protective inhibitory effects that nicotine has on T-helper 2 cell function [
74].
Immunological abnormalities in IBD patients result in epithelial damage. The main factors implicated in epithelial damage are abnormal mucus production and defective reparative mechanisms. Further expansion of inflammation is driven by intestinal flora with the involvement of immune cells that infiltrate into the lamina propria. Those cells include B-cells, T-cells, macrophages, neutrophils and dendritic cells. Immune regulation, which is a major factor for a correct immune response, remains defective, which leads to uncontrollable inflammatory response [
79,
80]. Consequently, high levels of proinflammatory cytokines in local tissues, such as TNF-a, IL-1b, interferon gamma and cytokines of the IL-23/T-helper 17 (Th17) pathway, are produced [
81,
82].
Oxidative stress (OS) may lead to damage of the mucosal layer and bacterial invasion, stimulating the immune response that initiates IBD [
83]. Although detailed pathways in IBD remain unclear, OS could be activated by environmental factors that include chemotherapy, radiation, smoking, drugs and alcohol [
84]. High alcohol intake directly impairs the mucosal barrier, resulting in IBD [
85]. High iron and copper intake, as well as fatty acids, increase the level of OS in the gastrointestinal tract [
86]. Other chemical substances like acrylamide, a chemical included in snacks, may trigger OS that leads to IBD [
87]. Conversely, diet-derived antioxidants can modulate inflammation and gut microbiota [
88]. Phytochemicals interact with the intestine by ameliorating oxidative stress, regulating cytokines, modulating gene expression and signaling to restore intestinal permeability [
89,
90]. By exerting antioxidative and antimicrobial actions, they may affect the structure of gut microbiota [
91,
92,
93,
94].
3.2. Current Practice for IBD Treatment
IBD’s severity can vary between individuals, and complications such as bowel perforation or obstruction, failure to thrive and increased cancer risk are among the notable ones. Therapy for IBD comprises medical agents/pharmacotherapy that include aminosalicylates (mesalazine or sulfasalazine), corticosteroids (prednisone), immune system modulatory agents (azathioprine), cell therapy, exosome therapy and biologic agents/biologics (infliximab), with surgical intervention reserved for complications of IBD or as a therapeutic modality when necessary [
95,
96,
97,
98,
99]. General measures such as lifestyle changes, dietary interventions and patient education on IBD as a disease can improve outcomes. Recent advances in biologics and new therapeutic strategies aim to achieve remission and prevent relapses. By modifying the disease course, a new concept is to promote mucosal healing as a treatment goal. This can be monitored by measuring specific biomarkers and by endoscopy. Several studies have demonstrated the benefits of these approaches with improved overall outcomes for patients [
97,
100].
Some patients can be non-responders to one or more of these pharmacological agents and even stop responding secondarily, after initial successful use of a specific agent. Side effects induced by the usage of the aforementioned agents can be rather severe [
100]. Therefore, discovering novel modalities for the treatment of IBD remains a fervent field of research [
101].
4. Antioxidant and Anti-Inflammatory Pathways Targeted by Phytochemicals for the Treatment of IBD
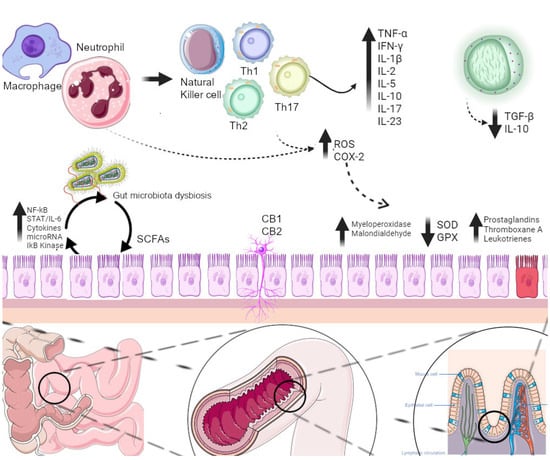
Phytochemicals and plant extracts target activated inflammatory and OS pathways in patients suffering from IBD (Figure 6). These substances seem to reverse or counterbalance the activated damaging pathways in various ways and are presented separately for each compound.
Figure 6. Various inflammatory and oxidative stress pathways activated in patients with IBD. Phytochemicals and plant extracts target these pathways. CB1-2: cannabinoid receptor 1-2, COX-2: cyclooxygenase-2, GPX: glutathione peroxidase, IFN-γ: interferon gamma, IL: interleukin, NF-kB: nuclear factor kappa B, ROS: reactive oxygen species, SCFAs: short-chain fatty acids, SOD: superoxide dismutase, STAT: signal transducer and activator of the transcription factor, TGF-b: transforming growth factor b, Th1-17: T helper 1-17 cells, TNF-a: tumor necrosis factor a.
Plantago ovata seeds are used as a form of dietary fiber, also known as
Ispaghula seeds [
25]. These are fermented and degraded by the anaerobic bacteria along the intestine to yield large amounts of short-chain fatty acids (SCFA), mostly butyrate and acetate. Diminished b-oxidation of luminal butyrate deprives the colonic microbiota of its preferred fuel and also results in energy deficiency of colonic epithelial cells. This has also been implicated in the pathogenesis of UC, as shown in rat studies [
102]. Butyrate also inhibits the production of some cytokines and lowers the transcription of the nuclear factor kappa B (NF-kB) factor while improving the health of the gut microbiota [
103]. Cancer protective effects of butyrate have also been described in a rat model [
104]. These effects have also been researched in butyric acid supplements that can modulate the gut microbiota but were deemed mostly ineffective on IBD patients as shown in several studies [
105,
106,
107]. Since butyric acid is not strictly a phytochemical, although it is a product of fermentable plant fiber [
107].
Plant essential oil supplementation to lower prostaglandin E2 and thromboxane A concentrations in the gut has been investigated [
27]. Increased concentrations of these inflammatory eicosanoids in biopsies of active UC patients have been documented. The arachidonic acid inflammatory pathway resulting in the production of prostaglandins, thromboxane and leukotrienes has been associated with UC [
108]. Thus, gamma-linolenic acid found in evening primrose essential oil that lowers arachidonic acid release was selected to target this pathway [
109].
The regulation of pathways of lipid metabolism or oxidative stress in immune-mediated diseases by microRNAs is a field of research receiving a lot of attention. In IBD, microRNAs are implicated in intestinal barrier function regulation as well as inflammatory pathways such as the NF-kB, the signal transducer and activator of the transcription (STAT)/IL-6 and Th17 signaling pathway. Phytochemicals have been shown to modulate microRNA expression [
110,
111]. Mastiha is a plant extract with anti-inflammatory and lipid lowering properties [
112]. The results after using it as a supplement in IBD patients indicate that it appears to regulate circulating levels of microRNA-155, a critical player in the differentiation of Th17 cells and other pro-inflammatory cells responding to TNF-a and interferons [
19].
Berberine has anti-inflammatory effects as described in animal studies, along with antioxidant and anti-cancer properties [
113,
114]. Another interesting property, also found in other bioactive phytochemicals of interest in IBD, is that it maintains high concentration inside the lumen of the gut with low systemic absorption. In contrast, another study showed that berberine did not alter gut tissue inflammation biomarkers COX-2 and NF-kB or cell proliferation marker Ki-67. As expected, plasma cytokine levels like TNF-a, IL-6 and IL-8 were also not altered by berberine administration [
45].
Anthocyanins that have anti-inflammatory and antioxidant properties seem to attenuate the course of colitis in animal models [
115,
116,
117]. Macrophages and neutrophils produce ROS in the inflamed bowel in IBD. The antioxidant system of the intestine is overwhelmed and thus potential antioxidants, such as anthocyanin rich compounds, have been investigated for their potential therapeutic effect.
Resveratrol, a polyphenolic flavonoid substance, has antioxidant and anti-inflammatory effects as verified by experimental models for colitis, where oxidant/antioxidant balance appears to play an important role in pathogenesis [
118]. Several articles indicate that resveratrol decreases oxidative stress, increases antioxidant enzyme concentration in tissues and decreases inflammatory biomarkers in animal models of UC [
119]. Other experimental studies showed that resveratrol reduces colonic tissue malondialdehyde and myeloperoxidase but increases superoxide dismutase (SOD) and glutathione peroxidase (GPX) activity while it inhibits collagen I synthesis in IGF-1-stimulated fibroblasts [
120,
121].
Polyphenon E—EGCG is a part of the catechin family of polyphenols contained in green tea. This family of phytochemicals has been extensively studied in regard to anti-inflammatory and antineoplastic effects due to its potent antioxidant properties. EGCG has an effect in preventing NF-kB nuclear translocation by the inactivation of IkB kinase. This suppresses production of cytokines, inflammatory enzymes and inflammatory protein kinases while upregulating anti-inflammatory pathways [
122,
123].
Procyanidins and other phenols in Pycnogenol
® display various biomodulating, antineoplastic and antioxidant effects such as inhibition of NF-kB and cyclooxygenase stimulation of nitric oxide (NO) synthase and antihypertensive effect by inhibition of angiotensin-converting enzyme [
124,
125,
126]. It was shown that it increased SOD and GPX antioxidant enzymes while it reduced oxidative damage to proteins and lipids. It also increased DAO after 5 and 10 weeks of Pycnogenol
® administration. DAO is found primarily in the mucosa of the gut and is a marker of histamine intolerance and also of disease activity. It inversely correlates with small intestine barrier permeability [
51].
Silybinin is the main component of silymarin flavanolignans, the extract of a species of thistle (
Silibum marianum). Reports from experimental murine and rat models of colitis provide evidence for immune-mediated intestinal wall healing with reduction in TNF-a, NF-kB and intereleukin-1b and also in disease activity [
127,
128,
129].
Tormentil extract contains tannins, mainly agrimoniin, pedunculagin, laevigatin B and F found in the
Tormentillae rhizoma. The mechanisms of its actions are not fully understood. However, tannins are known to promote wound healing and they have anti-inflammatory and anti-secretory results while their antibacterial properties may regulate the gut microbiota [
46].
T2 is a chloroform/methanol extract of
Tripterygium wilfordii containing triptolide. It is used in China for a variety of immune mediated disorders such as rheumatoid arthritis. Triptolide inhibition of NF-kB and TNF-a signaling pathways, suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17 has been proven in experimental animal models of colitis [
130,
131,
132]. Regulatory T-cells modulate the immune response in the intestine by secretion of inhibitory cytokines such as IL-10 and transforming growth factor-b (TGF-b). Their disruption can affect CD and T2 can upregulate Foxp3+ CD4+ T-Cells [
52]. It should be mentioned that the safety of
Tripterygium wilfordii Hook F. remains a matter of significant debate and ongoing research [
133].
Ecabet sodium is a pine resin derivative phytochemical. Its anti-inflammatory effects are thought to come from its affinity to adhere to gastric mucosa, epithelial cells and ulcer regions. It enhances defensive factors of the mucosa such as NO systems, prostaglandins like PGE2 and the mucin pathway whose depletion is related to UC pathogenesis [
134,
135,
136,
137].
Herbal preparations used as IBD complementary or alternative therapies are being utilized more often. An interesting example can be made out of the herbal combination of myrrh, chamomile flowers and coffee charcoal, which has been used for 50 years as a simple remedy for diarrhea. After considering the individual constituents and their known and validated effects from animal and experimental studies, it was concluded that the herbal preparation ought to target multiple pathways. Furanosesquiterpene, commiporic, commiphorinic acids and volatile oils contained in myrrh resin have demonstrated anti-inflammatory and antibacterial effects in animal studies. Inhibition of leukotriene production is hypothesized as the substrate. Chamomile tea or dry extract contain flavonoids, hydroxycoumarines and many other phytochemicals which are known for their anti-inflammatory, spasmolytic and antimicrobial properties. Finally, coffee charcoal is used for its antibacterial and absorbing properties. After confirmation of the preparation’s beneficial effect, further clarification of individual phytochemical bioactive substances and development can be undertaken [
32].
Cannabis consists of many phytochemicals like alkaloids, glycoproteins, terpenoids, flavanoids and cannabinoids. Among them, cannabidiol, tetrahydrocannabinol and cannabigerol are the most investigated bioactive substances for various diseases [
138,
139]. Cannabidiol and tetrahydrocannabinol interact with the endocannabinoid system to produce their effects. Cannabinoid receptor 1 (CB1) and 2 (CB2) are found in the brain but also in the submucosal and myenteric nerve plexus of the intestine, with the latter also appearing in immune system cells [
140]. Cannabinoids also target various other receptors in a complex process that must be further clarified. Endogenous and phytochemical cannabinoids acting as CB2 agonists or fatty acid amide hydrolase inhibitors display reduced inflammation in colitis animal models as demonstrated by recent studies [
141]. CB2 also appears to be upregulated in UC patients [
142].
Inulin-type fructans or FOS have been suggested to be effective in human IBD as prebiotics, ultimately enhancing the gut microbiota by stimulating the growth in protective bifidobacteria and
F. prausnitzii populations. They are non-digestible polymers of fructose that in animal models of colitis decreased disease activity and decreased NF-kB. In addition, fermentation of such prebiotics produces SCFAs that enhance intestinal epithelial barrier integrity. Furthermore, in combination with FOS, bacteria such as
B. longum taken from healthy subjects appear to have a clinical benefit [
143,
144].
Curcumin is a bioactive phytochemical compound isolated from the well-known plant turmeric (
Curcuma longa). It has been used as a food additive or spice with antioxidant, anti-inflammatory and antineoplastic properties [
145,
146,
147]. In vitro experimental studies on human colon cell lines provide evidence that curcumin inhibits carcinogenesis and inflammation through reduction of NF-kB, cytokines and cyclooxygenase-2 activation [
148]. Similar findings have been found in animal model studies [
149,
150].