1. Epidemiology, Risk Factors, and Clinical Course of Atherosclerosis in Arterial Territories outside Coronary Arteries, with a Particular Emphasis in Elderly Patients
According to data from the Central Statistical Office, there were nearly 10 million people in Poland over the age of 60 years in 2021, including approximately 120,000 individuals aged over 90 years. This number is constantly increasing due to advancements in medical technology, better living standards, and increased awareness regarding health in society. With time, the prevalence of atherosclerosis also rises, meaning that the management of elderly patients with this condition is becoming increasingly challenging.
Patients with atherosclerosis in one of the arterial areas following MI, limb ischemia, or cerebrovascular accident have a high risk for the coexistence of atherosclerotic stenoses in other arterial areas [
78,
79]. Epidemiological studies indicate that among middle-aged patients admitted to angiography with one-territory atherosclerotic disease, about 1.5% have arterial occlusive lesions in all arterial beds (CAD + ICAS + PAD, and/or RAS) [
78]. This number increases to 4% in patients aged 80 years old [
79]. The relative risk of having at least two-territory atherosclerotic occlusive disease is 15.7-fold higher in patients with claudication; 2.1-fold in patients with multivessel CAD; 2.8-fold for serum creatinine level >1.3 mg/dL; and 1.9-fold, 2.4-fold, and 2-fold in patients with hyperlipidemia, smokers, and women, respectively [
78]. One ischemic event leads to another in the same or another arterial area [
46,
80,
81]. In the EXSCEL trial including 14,751 participants; 26.5% were without atherosclerosis, and 58.9% had one-bed, 12.3% had two-bed, and 2.3% had three-bed disease [
82]. An increasing burden of atherosclerotic disease was associated with increasing risk of major adverse cardiac events (HR, 1.71 [95% CI 1.46–2.02]; 2.61 [2.17–3.15]; and 3.46 [2.69–4.45] for one, two, and three beds, respectively,
p < 0.001 for all) and all-cause mortality (1.94 [1.56–2.42]; 3.03 [2.33–3.95]; and 3.66 [2.59–5.18] for one, two, and three beds, respectively,
p < 0.001 for all) [
82]. In line, in the REACH registry including 23,985 participants, one-year outcomes of the composite cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke event rate was 4.3% for the overall population and highest in patients with triple-bed disease (9.9%) [
83].
Its worth noting that clinicians typically treat only the initially affected territory, mainly CAD, without consideration of the other affected territories, and they may lack awareness of the overall atherothrombotic syndrome [
84].
Atherosclerosis in coronary and non-coronary arteries is stimulated by the same cardiovascular risk factors (i.e., age, male, cigarette usage, diabetes, hypertension, dyslipidemia, and obesity) [
46,
85,
86]. However, in the case of ICAS, the dominant factor is hypertension, while in coronary arteries, the dominant factor is hyperlipidemia. In the Framingham Heart Study, for every 20 mmHg increase in systolic BP, there was a doubling in risk of developing ICAS greater than 25% [
49,
57]. Smoking and diabetes are the main factors in PAD [
87]. A meta-analysis of epidemiological studies from the USA, including a total of over 38,000 participants covered by the primary prevention program for heart and vascular diseases, showed that cigarette smoking and diabetes increase the risk of developing PAD by 4.0–5.5 times and that this risk is 10 times greater in females when compared to males [
86]. Diabetes is associated with additional risks for PAD patients such as diabetic foot, diabetic angiopathy, sensory neuropathy, and hyalinization of the intima of small arteries below the knee [
88,
89]. Furthermore, in diabetes, microangiopathy coexists with macroangiopathy, amplifying cardiovascular risk in the patient [
86]. Thus, for patients with diabetes, to obtain more systematic clinical care, comprehensive diabetes care centers focusing on panvascular diseases are required [
90].
2. Familial Hypercholesterolemia and the Extent of Atherosclerotic Lesions
Familial hypercholesterolemia (FH) is a monogenic, autosomal dominant disorder that from birth results in elevated low-density lipoprotein cholesterol (LDL-C) and markedly increased risk of premature atherosclerosis [
91,
92]. In this disorder, the metabolism of LDL-C is altered through mutations in the gene for LDL receptor (LDLR) and less commonly in those for apolipoprotein B (APOB), proprotein convertase subtilisin-kexin type 9 (PCSK9), and others [
93]. In most FH cases, the mean LDL-C level exceeds 190 mg/dL in adults and over 160 mg/dL in children [
94]. According of the Dutch Lipids Clinics Network (DLNC) criteria, FH is diagnosed in 1 out of 250 adults [
95]. The increased LDL-C levels since childhood are associated with a premature manifestation of atherosclerotic disease. The risk of developing CAD in FH patients is approximately 13-fold higher than in the general population [
96]. CAD is evident in patients with FH from the age of 17 in men and the age of 25 in women, and up to 25% of the adolescents with FH present coronary artery calcification and/or aortic valve calcifications [
97]. In a study that included a representative sample of adult patients with hypercholesterolemia in outpatient clinics in Poland, FH based on DLNC criteria was diagnosed in 3.6% of the examined patients [
97].
Despite the common belief that atherosclerosis extent is greater and more severe when hyperlipidemia begins at very early stage of life, patients with FH atherosclerotic involvements differ tremendously in terms of arterial territories. Atherosclerotic plaques causing significant obstructive arterial disease mainly affect coronary and lower limb arteries [
98,
99]. Conversely, in carotid arteries, FH is associated with the increased carotid intima–media complex thickness in common and internal carotid arteries, but it is less likely to cause a significant arterial lumen narrowing [
100]. In this line, atherosclerotic plaques are more prevalent in renal arteries in FH, but rarely obstructive, as compared to patients without FH [
101]. However, the small number of available studies, as well as their characteristics (sample size, diagnostic criteria used, retrospective or cross-sectional design) limits the evidence for association between FH and stroke, PAD, or RAS [
102]. In conclusion, FH is associated with a substantial excess mortality from CAD in young adults but may not be associated with a substantial excess mortality in older patients [
102,
103].
3. Lower Extremity Peripheral Arterial Disease
PAD is the third leading cause of atherosclerotic morbidity, following CAD and stroke. PAD affects >230 million adults worldwide and is associated with increased risk of various adverse clinical outcomes [
104,
105]. Every year in Poland, there are approximately 40,000 hospitalizations due to PAD and over 9000 amputations related to this disease [
106]. Various diagnostic methods for detecting PAD have led to conflicting results in epidemiological studies [
107,
108,
109,
110]. Some studies suggest that males are slightly more prone to develop PAD than females. A meta-analysis conducted by Lin et al. revealed that the prevalence of PAD is 6% in males and 5% in females aged between 60 and 69 years; the prevalence is 11% and 9% in those aged between 70 and 79 years; and 26% and 21% are over 80 years of age, respectively [
107]. The Rotterdam study, which was published in 1998 and based on ankle-brachial index (ABI) measurement, revealed that the incidence of PAD was greater in females than in males, with 20.5% of females and 16.9% of males affected [
107]. There is a clear increase in incidence with age, ranging from 6.6% in males and 9.5% in females aged 55–59 years to 52.0% in males and 59.6% in females over the age of 85 years [
107,
108,
109,
110].
Such a high frequency of PAD in the elderly is not necessarily correlated with the frequency of intermittent claudication, which affects only 6.0% of males and 2.5% of females at the age of 85 years [
109]. This is due to adaptive changes occurring in the limb arteries, such as the slower dynamics of plaque growth, positive vascular remodeling, and the development of collateral circulation (
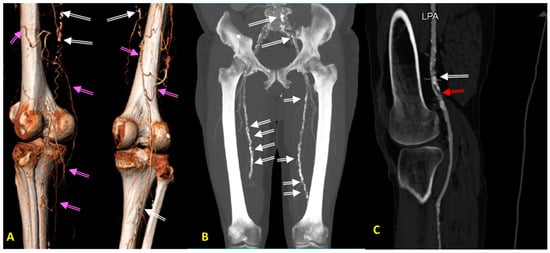
Figure 1). Furthermore, older people with PAD tend to present with atypical symptoms such as general fatigue, leg weakness, tenderness to touch, hypoesthesia, and edema, rather than intermittent claudication. It is also worth noting that symptoms of neuropathy, sciatica and other root pain syndromes, venous thrombosis, and muscle atrophy during lipid-lowering therapy can often mimic PAD symptoms and should, therefore, be considered in the differential diagnosis.
Figure 1. (A–C) Diagnostic images of peripheral arterial disease (PAD) in patients with advanced atherosclerosis, obtained with computed tomography angiography (CTA). (A) CTA image obtained in a 73-year-old hypertensive man with asymptomatic PAD showing long occlusions of both superficial femoral arteries (SFA). Please note a very well developed collateral circulation (pink arrows) from deep femoral arteries and white spots along both SFAs corresponding to calcifications (white arrow). (B) CTA image obtained in a 78-year-old hypertensive man with moderately symptomatic PAD showing occlusions of both SFA in the Hunter channel. Please note a very excessive calcifications in iliac and femoral arteries (white arrows). (C) CTA image obtained in a 78-year-old hypertensive woman with type 2 diabetes, as well as a critical PAD causing rest pain resulting from ruptured calcified plaque (white arrow) in the left popliteal artery (LPA), with a recent vessel thrombosis below the calcified plaque (red arrow). Please note the lack of collateral circulation.
In an outpatient setting, the probability of PAD in individuals who are under the age of 80 years can be estimated using the online calculator (
http://ckdpcrisk.org/padrisk, accessed on 9 January 2024). It is difficult to predict the risk of incidents and death in PAD patients due to its poor symptomatology. However, after PAD diagnosis, an estimated 20% of patients may experience MI, stroke, or death within the first 5 years. Critical ischemia of the lower limb resulting in amputation has the highest risk of death, and after amputation, the expected average survival time for 60% of patients is 2 to 5 years [
106,
107,
108,
109,
110].
Smoking is the greatest modifiable risk factor for the development and progression of PAD. According to Meijer et al.’s study, smoking accounted for most occurrence of PAD cases with odds ratio of 2.8 (95% CI, 2.3–3.4,
p < 0.001) [
84]. Tobacco smoke increases endothelial cell permeability by the formation of reactive oxygen species, which enables atherosclerosis to settle within vessel walls [
111].
It is important to note that patients aged 65 years with PAD (ABI < 0.9) have a 2.43-fold greater risk of death (95% CI: 2.18–2.72) compared to those without PAD (ABI ≥ 0.90). However, as PAD patients age beyond 65 years, mortality rates decrease with an average reduction of 13% (95% CI: 0.83–0.92) for each additional 5 years [
107]. The authors of the study concluded that with increasing age, PAD gradually ceases to be a risk factor for death when compared to the same age group without PAD [
107].
The results of a Japanese cohort study, which followed 3122 PAD patients over 65 years of age, showed that the number of cardiovascular risk factors is an important parameter determining the survival of patients [
110]. In this study, the accumulation of three cardiovascular risk factors (diabetes, hypertension, hyperlipidemia) occurring in 419 (13.4%) patients increased the risk over threefold for both MACCE (HR: 3.06; 95% CI: 2.45–3.82;
p < 0.0001) and critical lower limb ischemia (major adverse limb event (MALE): HR: 3.22; 95% CI 2.39–4.35;
p < 0.0001). The age group itself (65–74 years, 75–84 years, >85 years) was not a risk factor for MACCE; however, greater age was a protective factor against the occurrence of MALE, which is associated with cessation of inflammatory-thrombotic processes in the lower limbs. Both middle-old seniors (75–84 years old) and oldest-old seniors (>85 years old) had a lower risk of MALE (HR 0.79; 95% CI 0.66–0.96, respectively;
p = 0.0150 and HR: 0.53; 95% CI 0.36–0.77;
p = 0.0010) when compared to the group of youngest-old seniors (65–74 years old) [
110].
Sykora et al. assessed the risk of MACCE in more than 22,000 outpatients with PAD according to age structure (<50, 50–59, 60–69, ≥70 years) and ABI (<1.0; 1.0–1.4; >1.4) [
112]. MI and stroke were significantly more common in PAD patients diagnosed before the age of 60 years (10%) compared with patients whose PAD was diagnosed after the age of 70 years (6.8%) (HR 2.33; 95% CI 1.95–2.78) [
112]. Similarly, the risk of MALE and amputation was lowest in the group of patients over 70 years of age and gradually increased in the groups of younger patients. It is puzzling that the causes of death were not analyzed in this study, whereas the mortality rate after 2 years of observation was 51% [
112].
Another study investigated the factors that influence the risk of death in PAD patients over the age of 80 and 90 years [
113]. One-year mortality was 25% (35/123 patients) above the age of 80 years compared to 10% (13/123 patients) in the group of patients under the age of 80 years. In the group of PAD patients aged 80 years and above, it was found that the concentration of cardiovascular markers such as troponin > 40 ng/L (HR: 4.6; 95% CI: 1.4–15.3) and NT-pro-BNP > 450 pg/mL (HR: 3.9; 95% CI 1.8–8.8) and the occurrence of MALE (HR: 3.1; 95% CI 1.6–5.9) were strong predictors of patient death [
113]. Features of ischemia, HF, and MALE determine the survival of this group of patients.
The coexistence of atherosclerosis and diabetes is associated with the worst short- and long-term prognosis and poses significant problems in the diagnosis and management of these patients. Diagnosis of PAD by measurement of ABI (class I-C of the recommendations for PAD diagnosis) is characterized by a sensitivity and specificity of >90–95% when ABI < 0.9 [
114]. Ankle-brachial index < 0.5 is an indicator of severe limb ischemia. However, this indicator often fails in patients with diabetes or end-stage renal failure and in those being treated with dialysis [
114]. Hyalinization of the vessel intima leads to a falsely high ABI (above 1.4–1.5), making PAD diagnosis difficult. Atherosclerosis of small vessels in the tibial, peroneal, or dorsal foot and metatarsal arteries makes the treatment of these patients very difficult and is a challenge for vascular surgeons. In these patients, the toe-brachial index (TBI) is assessed using a small cuff placed on the big toe and an ultrasound (class of recommendation: I-C). A TBI value < 0.7 is diagnostic for PAD [
106]. In elderly patients, due to the frequent coexistence of diabetes and renal failure, the TBI index should be used more often to determine the prognosis.
According to most scientific societies, in-depth diagnostic imaging (duplex Doppler ultrasound, angio-CT, angio-MRI, angiography) of the type, location, and severity of stenoses in the lower limb arteries should only be performed in elderly patients with features of critical or acute lower limb ischemia. This diagnosis should be justified by the need for revascularization of the lower limb artery or arteries in patients with ulceration or trophic changes and in those who are at risk for amputation [
115]. Imaging tests with contrast administration are particularly problematic in the elderly, who are characterized by multimorbidity and are associated with the risk of renal function deterioration, up to and including dialysis [
115].
Patients presenting with symptoms of MALE, as well as with ischemic rest pain (Fontaine class III symptoms), ulceration or gangrene (Fontaine class IV), or Rutherford class 4–6 symptoms, require revascularization to save the limb. This is particularly relevant in non-healing ulcers despite previous treatment. If the systolic BP in the toe is less than 80 mmHg, the likelihood of healing the ulceration is low [
106,
107,
116]. Treatment may be considered on an individual basis in patients with short-distance isolated arterial stenosis with significant distance limitation (claudication in rest or after short-distance < 50 m, no improvement after hardening with walking—according to Rutherford class 3) [
107].
Acute limb ischemia unrelated to atherosclerosis should always be kept under consideration [
116]. Ischemia of the lower limb may be caused by embolism of the femoral artery, popliteal artery, or tibiofemoral trunk (as in the course of atrial fibrillation), or by vegetation from the heart valve or left atrial myxoma. Such patients require urgent embolectomy regardless of their age.
4. Renal Artery Atherosclerosis
Renovascular disease (RVD) due to atherosclerotic uni- or bilateral RAS is a disease of the elderly and generally involves the proximal segment of the renal artery. Thus, in the elderly, RVD is a de novo phenomenon, and the plaque is considered as ‘young’. The significance and treatment of RAS are some of the most controversial issues. RAS is predominately an age-dependent pathology, having a prevalence of less than 1% in the general population. However, above the age of 65 years, its incidence is 7%, while above the age of 75 years, it can be found in approximately 40% of examined patients [
117].
If RAS significantly reduces renal perfusion and causes renal ischemia, it leads to activation of the renin–angiotensin–aldosterone (RAA) axis with the following consequences: increased peripheral resistance, water and sodium retention, hypervolemia resulting in the development of hypertension, myocardial hypertrophy, and accelerated atherosclerosis. The consequences of RAS include MI, stroke, aortic dissection, and pulmonary edema [
118,
119,
120]. A cardiac manifestation of RAS may be circulatory failure. In its acute form, RVD manifests as pulmonary edema with preserved left ventricular systolic function (pulmonary flash edema), while in the chronic form, this is seen as left ventricular HF. Chronic renal ischemia results in nephropathy and progressive failure, combined with renal atrophy up to and including cirrhosis [
118,
119,
120]. In unilateral RAS, prolonged stimulation of the RAA axis; increased sympathomimetic activity; oxidative stress; and activation of endothelin, thromboxane, and 20-hydroxyeicosatetraenoic acid (20-HETE), with a concomitant decrease in prostaglandin and nitric oxide production, can lead to inflammation, fibrosis, and progressive failure of the second kidney [
121]. Historical studies show that the four-year survival rate of RAS patients with over 75% stenosis is 68%, while those with 95% stenosis is 48%, compared to 89% in patients without this pathology [
122].
RAS is the most common cause of secondary hypertension, so it is not surprising that it can be detected in approximately 2–5% of hypertensive patients and in 30–40% of patients with coexisting hypertension and renal failure. Moreover, RAS is also found in 30–50% of patients with congestive HF. Finally, RAS increases the risk of ischemic heart disease and PAD by 4 times, HF by 3.5 times, stroke by 3 times, and death by 2.6 times [
123]. In patients with coronary artery disease (CAD), the incidence of RAS is approximately 30–33%, of which 7–15% of these patients have a stenosis of greater than 50%. Bilateral stenosis is found in 4–11% of patients [
118,
124]. The prevalence of RAS is estimated to be as high as 40% in patients with PAD and as high as 50% in patients with multilevel atherosclerosis [
125,
126]. It is noteworthy that in the group of patients with incidentally detected RAS at the moment of diagnosis, 65.5% had hypertension and 27.5% had renal failure [
127].
Atherosclerotic RAS can be suspected in patients with a sudden onset of hypertension over 50–55 years of age [
128]. Additionally, this should be suspected in essential hypertension patients who have a worsening of previously well-controlled BP [
128]. Other features that may indicate the presence of RAS are unexplained renal failure or its onset after the initiation of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or sodium-glucose cotransporter-2 inhibitors (SGLT2i); a difference in the size of kidneys by more than 1.5 cm; resistant or malignant hypertension; recurrent pulmonary edema with preserved left ventricular contractility; or symptoms of multilevel atherosclerosis [
128]. Age, female sex, hypertension, presence of generalized atherosclerosis, multivessel CAD, diabetes, and elevated creatinine and LDL cholesterol levels have all been identified as independent risk factors for RAS in multivariate analysis [
129,
130].
Diagnosis for RAS can be started with ultrasonography of the renal arteries (Class IB recommendation). Compared to the carotid or lower limb arteries ultrasonography, this examination is more challenging, as it requires excellent equipment and an experienced examiner. However, it allows for accurate RAS detection exceeding 60% with a sensitivity and specificity of 98%, as well as with a positive predictive value of 99% and a negative predictive value of 97% [
131]. Among the most important ultrasonographic parameters that could indicate the presence of RAS are peak systolic velocity (PSV) and diastolic velocity at the stenosis site exceeding 2.0 and 0.5 m/s, respectively, and the renal-aortic ratio (RAR) greater than or equal to 3.5 at the stenosis site [
131,
132]. The examination is complemented by parameters of renal size, structure, and assessment of intrarenal flow. Preoperative assessment of the degree of RAS is essential for determining the treatment options (
Figure 2). CTA is characterized with high sensitivity; however, CTA carries a risk of radiation exposure, requires exogenous contrast agents that are potentially nephrotoxic, and cannot be performed in patients allergic to iodine contrast agents [
133,
134]. In contrast, MRA has the benefits of no ionizing radiation, high repeatability, and a low incidence of adverse reactions with gadolinium contrast agents [
134]. MRA for the preoperative examination of the kidney that has been increasingly studied as an MRI technique has continued to advance [
135]. Both CTA and MRA have known limitations as they tend to overestimate the degree of stenosis and, in addition, large calcifications make it difficult to assess the degree of stenosis. On the other hand, renal perfusion scintigraphy and renin activity in plasma or renal veins, which were commonly used years ago, are not currently recommended as diagnostic methods (Class III C recommendations) [
136].
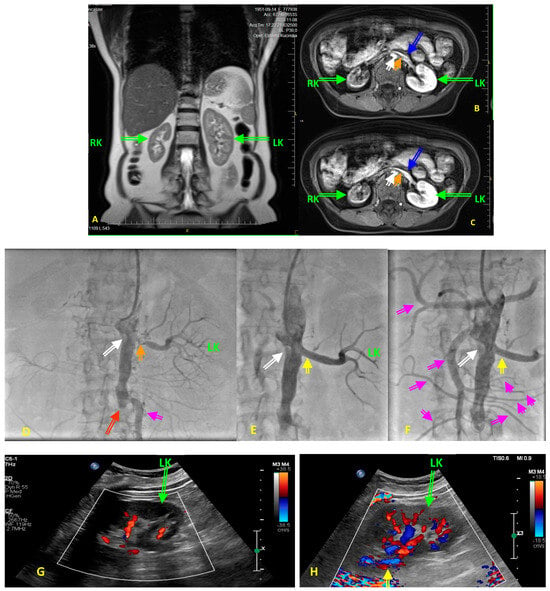
Figure 2. (A–H) Diagnostic images obtained in a 73-year-old hypertensive woman with type 2 diabetes before renal artery angioplasty with stent implantation (PTA) for the left renal artery stenosis (RAS). The patient was referred to renal PTA because of resistant hypertension, with blood pressure values above 200/120 mmHg despite five blood-lowering agents in maximally tolerated doses, after the patient developed acute renal failure following treatment initiation with empagliflozin. At the six-month follow-up period following PTA, the patients was still on five blood lowering agents with blood pressure values not exceeding 150/75 mmHg, with a stable renal function with an eGFR of 27 mL/min/1.73 m2. (A) Pre-intervention diagnostic magnetic resonance angiography (MRA) showing quantity alterations in right kidney (RK) and left kidney (LK) dimensions and unequal renal parenchymal signal intensity. (B,C) Transverse scans of the aorta, renal arteries, renal veins (blue arrows), and both kidneys on MRA. Images display, in early and late gadolinium-contrast phases, the small size of the RK, low signal in the RK, and unclear corticomedullary boundary in both kidneys. Please note the intensive calcifications in the abdominal aorta (white arrow), and the critical non-calcified, presumably soft ostial lesion of the left renal artery (orange arrow). Perfusion of the LK is preserved as yet, while the RK shows features of cirrhosis. (D) Renal catheter angiography confirmed an ostial 95% left RAS (orange arrow), and absent right renal artery (white arrow). Thus, the patient is presented with a stenosis of a single functional kidney. Please note the total occlusion (red arrow) of the distal part of abdominal aorta and both common iliac arteries. Pink arrow indicates collateral pathway. (E) The final result of PTA in renal artery (yellow arrow). Please note that the PTA procedure was performed via a transradial access route due to the total occlusion of the distal part of abdominal aorta and both common iliac arteries (Leriche’s syndrome). Pink/violet arrow indicates the large collateral artery providing blood supply to the pelvis and lower extremities. White arrow is for the total occlusion of the right renal artery. (F) Final angiography of aorta displaying numerous collaterals (pink arrows) from cephalic trunk, mesenteric arteries, to abdominal and pelvis organs. Yellow arrow indicates the final effect of RAS procedure, whereas white arrow desplays occluded right renal artery. (G) Color Doppler ultrasound showing reduced intrarenal flow in the LK before PTA. (H) Color Doppler ultrasound showing restoration of the intrarenal flow in the LK after PTA, yellow arrow indicates the final effect of RAS procedure.
Non-surgical management of patients with RAS is identical to that for atherosclerosis localized to other arterial areas and includes a rigorous control of risk factors through smoking cessation, diet, weight normalization, exercise, and pharmacotherapy to normalize BP, achieve target blood lipid levels, and manage diabetes [
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147].
In the case of RAS, the largest challenge is to determine whether the existing stenosis is an incidental finding or whether it significantly impairs renal perfusion and is responsible for the existing problems, therefore being an indication for intervention. This can be particularly difficult in elderly patients, as they generally have advanced atherosclerosis in various arterial areas; impaired renal function with reduced eGFR; and very often, long-term hypertension.
Currently, the treatment of choice for RAS is percutaneous transluminal angioplasty (PTA), generally combined with stent implantation, which significantly reduces the incidence of recurrent stenosis and improves long-term outcomes [
120,
141,
146,
148,
149]. It has a low complication rate of less than 5% in experienced centers and an acceptable stenosis recurrence rate of approximately 15% [
148,
149]. Invasive treatment aims to improve BP control and protect renal function, which should translate into an improved prognosis for the patient. The results of numerous observational studies (usually single-center) indicate an improvement in BP control in approximately 60–70% of patients with atherosclerotic RAS, although curative rates are only 5–15% of patients. Improvement in renal function is seen in approximately 25–30% of patients and is as common as a deterioration in renal function. In approximately 40–50% of patients, no significant effect of the procedure on renal function is observed, which can be interpreted as both a lack of effect and a favorable stabilization of renal function [
149,
150]. Randomized clinical trials (RCTs) have compared the effects of PTA and pharmacological treatment on BP control, renal function, cardiovascular incident rates, and patient survival. Of the eight completed RCTs, only the two small ASPIRE 2 and RENAISSANCE trials showed the superiority of PTA over pharmacological treatment, and this was only regarding BP, with no effect on renal function, MACCE rates, or survival [
150]. Other studies, including the two largest, the ASTRAL and CORAL studies, did not show an advantage of invasive procedure over pharmacotherapy in any of the parameters analyzed [
142]. Although a meta-analysis of four of these trials showed a significant improvement in diastolic BP and a reduction in the number and doses of blood-lowering drugs after PTA compared to pharmacotherapy, the lack of effect of PTA on prognosis in the RCTs remains a fact [
150].
As a result, the current guidelines allow PTA for atherosclerotic RAS only in cases of unexplained recurrent HF or sudden pulmonary edema and having the lowest possible recommendation level of IIb-C [
136]. Indeed, the mortality rate of patients with flash pulmonary edema at the six-year follow-up was 90%, and in cases of recurrent chronic circulatory failure, performing PTA reduces the number and frequency of hospitalizations and improves the NYHA class, which fully justifies this treatment method [
151,
152].
Factors that may indicate a potentially beneficial effect of the procedure include the short (less than 2 years) duration of hypertension, very elevated levels of BP, resistant hypertension, deterioration of renal function recently or after the initiation of ACEIs/ARBs, episodes of acute or chronic circulatory failure with preserved left ventricular contractility, high degree of stenosis, and bilateral stenosis or unilateral stenosis of a single functional kidney. Patients with suspected or established RAS should be referred to a specialist center with appropriate experience in the assessment of these patients and invasive treatment.
5. Carotid Artery Atherosclerosis
The prevalence of asymptomatic ICAS also increases with age. Stenosis above 50% was found in 4.8% (95% CI: 3.1–7.3%) of males and 2.2% (95% CI: 0.9–4.9%) of females under 70 years of age, compared with 12.5% (95% CI: 7.4–20.3%) in males and 6.9% (95% CI: 4.0–11.5%) in females over 70 years of age [
155]. Under 50 years of age, the incidence of ICAS > 50% is minimal (0.2% in females and 0.3% in males).
Histopathological findings from carotid artery atherosclerotic plaques clearly indicate that as the patient’s age increases, atherosclerotic lesions become larger, containing locally more inflammatory cells and MMP-9 and fewer smooth muscle cells (which normally stabilize atherosclerotic plaques) [
156]. These characteristics of carotid atherosclerotic lesion structure predispose the patient to symptoms of cerebral ischemia, the risk of which increases with the patient’s age. The result is that the risk of death and stroke in patients over 75 years of age is greater if the patient is only on pharmacotherapy compared to those with additional surgical treatment [
157]. This is true despite the current availability of effective pharmacotherapy [
156,
157,
158,
159,
160,
161].
The recommendations include pharmacotherapy, smoking cessation, and dietary modification in all patients with asymptomatic as well as symptomatic ICAS, as well as revascularization in selected groups of patients (endarterectomy (CEA) or PTA). Pharmacotherapy, as in other arterial areas, includes hypotensive, antiplatelet, hypoglycemic, and lipid-lowering agents. A prospective cohort of 101 patients with asymptomatic ICAS found lower annual risk of ipsilateral stroke events (AR = 0.34%, 95% CI 0.01–1.87) when patients were treated with antiplatelet agents, statins, and anti-hypertensive medications [
162]. A systematic review of 3724 patients with asymptomatic ICAS showed decreasing rates of stroke related to improvements in medical therapy [
163].
Despite medical treatment, asymptomatic ICAS exceeding 70% is associated with an annual risk for ischemic stroke of 2–4% (10–20% over 5 years) in the elderly [
157]. In contrast, the presence of neurological symptoms in patients without interventional treatment (CEA or PTA) increases the risk for developing stroke by 4–12% per year.
In contrast to other arterial areas, in ICAS, age does not reduce the risk of stroke and death but carries with it additional complications such as severe calcification (
Figure 3), vessel tortuosity, stiffness, low compliance, atherosclerosis, and deformation of the aortic arch, which should be considered when treating these patients surgically [
164,
165].
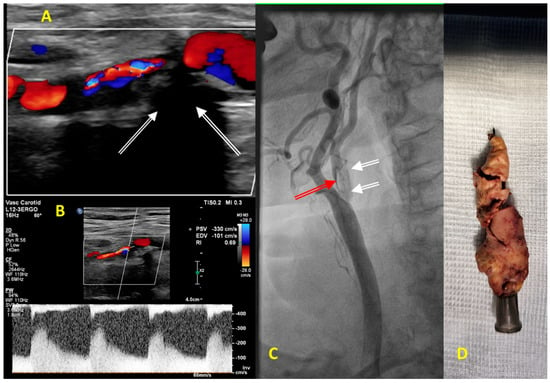
Figure 3. (A–C) Diagnostic images obtained in an 83-year-old man before carotid endarterectomy on the right internal carotid artery (RICA) stenosis. (A) Color Doppler ultrasound showing abnormal turbulent flow in the proximal segment of the RICA. Carotid plaque is hyperechogenic, massively calcified with a typical shadowing below calcifications (white arrows). (B) Pulse Doppler showing a significant increase in the peak systolic and the end-diastolic velocities of 330 cm/s and 101 cm/s, respectively, consistent with severe stenosis of the proximal segment of RICA that corresponded to lumen stenosis of more than 90%. (C) Carotid catheter angiography confirmed a 2 cm long 95% RICA stenosis (red arrow) with calcified morphology (white arrows). (D) The image displays massively calcified plaque excised from the RICA during carotid endarterectomy.
When deciding on surgical treatment in elderly patients, contraindications and factors increasing the risk of surgical complications should be taken into account [
164,
165]. Factors that increase the risk of complications from CEA include anatomical features of the stenosis (history of radiation and surgical treatment of neck tissue or previous CEA, tracheostomy, contralateral laryngeal nerve palsy, contralateral carotid artery occlusion, thrombus, stenosis of the distal ICAS or proximal common carotid artery) and the patient’s general condition (age > 80 years, MI in the last 30 days, NYHA class III and IV, severe renal failure or lung disease, recent percutaneous coronary intervention requiring antiplatelet therapy). Risk features associated with stent implantation include unfavorable anatomical conditions for stent insertion such as arterial tortuosity, lack of access site, inconvenient aortic arch, atherosclerosis/aneurysm/malformation of intracerebral arteries, severe carotid artery calcification, thrombus, age over 80 years, severe renal failure, stroke within the last 4–6 weeks, and contraindications to antiplatelet therapy [
164,
165].
When considering periprocedural complications of PTA and CEA in patients younger than 70 years, the risk of stroke, death, or MI was similar for both methods, while among patients over 70 years, the 30 day risk of stroke and death was elevated for PTA (OR 2.20, 95% CI 1.47–3.29) [
166,
167]. Based on this, current guidelines state that it is reasonable to consider patient age in choosing between CEA and PTA, as CEA is associated with improved outcomes compared to PTA for those over 70 years, while in younger patients, PTA is equivalent to CEA in terms of risk periprocedural complications, a Class IIa recommendation [
167]. In patients undergoing PTA or CEA for either symptomatic or asymptomatic ICAS, perioperative mortality, stroke rates, and other perioperative complications increase in those above 75 years of age, especially above 85 years of age, compared to younger patients [
167].
There are limited data on outcomes for frail individuals who undergo CEA or PTA. In a cohort study that included 37,875 patients, with an average age of 75, who underwent either CEA or PTA in the NSQIP database, 27% were frail [
168]. In total, 95.4% underwent CEA and 4.6% underwent PTA. Compared to those who were not frail, individuals identified as frail were more likely to experience poor outcomes such as a post-operative complication (OR 3.2 (2.1–4.8)
p = 0.01) and mortality (OR 2.1 (1.6–3.7)
p = 0.01), irrespective of the method used [
168].
At the same time, good surgical outcomes (CEA and CAS) have been documented in groups of older patients who were carefully prepared for surgery, with particular attention to renal function, anemia, and surgical technique [
169,
170,
171]. In a study conducted by four U.S. centers, the 30 day risk of death and stroke after percutaneous ICAS treatment was 2.8% (11/389) in a group of patients aged over 80 years (83.2 ± 2.8 years), of whom 2/3 were asymptomatic and 1/3 presented with symptoms of cerebral ischemia before the procedure [
171]. In a study involving 193 patients ≥75 years of age with symptomatic ICAS, the 30 day risk of death or stroke was 7%, while in asymptomatic ICAS, this risk was 1.7%, and finally, this risk was 1.9% in younger individuals [
172].
In summary, the morphological structure of atherosclerotic plaques varies in different vascular areas. The severity of risk factors contributing to the development of atherosclerotic lesions also varies. These various manifestations of atherosclerosis require accurate consideration of its treatment. However, the advanced age of a patient must not disqualify the patient from surgical or endovascular intervention, especially as life expectancy is increasing and will probably exceed 90 years in 2050 [
187].
This entry is adapted from the peer-reviewed paper 10.3390/jcm13051471