1. Importance and Multifaceted Nature of the Aquatic Environment
The aquatic environment constitutes any natural or artificial setting predominantly comprising water, encompassing oceans, seas, lakes, rivers, streams, wetlands, and subterranean water reservoirs. This environment encompasses both freshwater and marine ecosystems [
1]. Covering around 71% of the Earth’s surface, water consists largely of saltwater in the oceans, accounting for approximately 97.5%. Conversely, only about 2.5% of Earth’s water is freshwater, primarily distributed in rivers, lakes, and underground reservoirs. A minor fraction of this freshwater is readily accessible and suitable for human use [
2]. Despite this limited accessibility, the aquatic environment constitutes a substantial portion of the Earth’s surface, playing a pivotal role in the planet’s dynamics. Supporting a diverse array of species, ranging from microorganisms like phytoplankton and zooplankton to larger organisms such as fish, amphibians, reptiles, birds, and mammals, many exclusive to aquatic ecosystems, it is estimated that over 50% of all known species inhabit aquatic environments [
3]. These species contribute significantly to ecosystem equilibrium, offer sustenance, and enhance biodiversity. Additionally, aquatic ecosystems perform crucial functions like climate regulation through carbon dioxide absorption and oxygen release, water purification by filtering pollutants, the provision of habitats for commercially valuable fish species, and the facilitation of recreational activities [
4]. The aquatic environment experiences influence from diverse factors including water temperature, salinity, pH, nutrient concentrations, currents, and anthropogenic activities. Safeguarding and conserving the aquatic environment are imperative to ensuring the resilience of ecosystems and the welfare of both aquatic life and human communities [
5].
2. Antibiotic Resistance (ABR) in Aquatic Environments
Aquatic animals contribute to 17% of global animal protein consumption, with fish providing nearly 20% of per capita animal protein for over 40% of the world’s population. Global food fish consumption growth has outpaced the consumption of meat from terrestrial animal production sectors, except for poultry [
6]. Since 2001, global aquaculture has grown at a rate of 5.8% annually, driven by increased demand for animal protein in fast-growing economies. Asia is the major contributor, accounting for nearly 90% of global aquaculture production, with China alone contributing 61% in 2016 [
7].
The rising demand for animal source nutrition has led to increasing intensification in animal production systems, including aquaculture. The transitional period toward intensive production often involves the use of non-therapeutic antimicrobials to enhance growth and compensate for insufficient biosecurity and management practices. Antimicrobial use in terrestrial food-producing animals is already high and is expected to increase significantly by 2030, especially in fast-growing economies like BRICS countries (Brazil, Russia, India, China, and South Africa) [
8].
Antibiotic use in aquaculture serves multiple purposes, including disease prevention, growth promotion, and water quality management. Disease prevention and treatment are vital in densely stocked aquaculture systems, where antibiotics are used to control bacterial infections [
9]. Prophylactic antibiotic use helps mitigate known risks during activities like transportation or stocking. Additionally, subtherapeutic antibiotic doses can promote growth; however, these practices exert strong selective pressure, favoring the emergence and selection of antibiotic resistance (ABR) strains, with subsequent dissemination of ABR traits through different routes, such as food, feed, and the environment [
10].
Antibiotics such as macrolides have 12–16 lactone rings and are lipophilic with low water solubility. They are generally bacteriostatic and are used to treat respiratory tract, skin, and soft tissue infections [
11]. Beta-lactam antibiotics contain at least one beta-lactam ring. They interfere with cell wall synthesis and are active against a wide range of bacteria, making them widely used, with penicillin being a prominent member. In addition, sulfonamides, derived from a p-amino-benzene–sulfonamide functional group, are widely used, have a long decomposition half-life, and are commonly used in veterinary medicine [
12]. Tetracyclines are amphoteric and unstable in bases but stable in acids. They are extensively used in veterinary and human medicine, in agriculture, and as food additives. They pose environmental challenges and may not be fully removed by wastewater treatment plants. Quinolones are fat-soluble and resistant to various environmental conditions. They are used for treating infectious diseases and promoting livestock and aquaculture, entering aquatic environments through wastewater and direct discharge [
13].
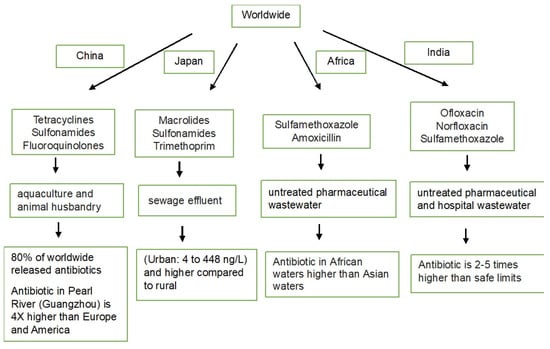
China is the world’s largest animal-feeding country, with expanding and intensifying operations. Hence, a significant number of antibiotics, approximately 53,800 tons out of the 92,700 tons used, are released into the environment, including surface waters, groundwater, and coastal waters [
14]. The most commonly detected antibiotics are tetracyclines, sulfonamides, and fluoroquinolones. Concentrations of antibiotics are generally higher in northern and eastern China, where the population density is higher. The main sources of antibiotics in water have been found to be wastewater from aquaculture and animal husbandry [
15]. The antibiotic concentration in the Guangzhou area of the Pearl River is 3–4 times that of rivers in Europe and America [
16]. A systematic review has mentioned that ABR isolates achieved nearly 92% of resistance towards antibiotics were detected in the region of China and have exceeded safe limits (Predicted No Effect Concentrations) in wastewater, treatment plant influents/effluents, and receiving environments. Wastewater and plant influent/effluent treatment plants have shown the greatest potential for resistance development. The tap and drinking water in the WPR and China have shown the highest levels and likelihood of exceeding PNECs, particularly for ciprofloxacin [
17].
On the other hand, macrolides, sulfonamides, and trimethoprim have been frequently detected in the water of the Tama River in the Tokyo metropolitan area with concentrations ranging from 4 to 448 ng/L. Antibiotic residues are generally higher in urban rivers compared with rural ones. The dominance of human antibiotics from sewage effluents has been noted to be a contributing factor to the antibiotic composition in urban rivers, rather than veterinary medicines from livestock wastewater [
18]. About 25% of wastewater treatment systems in Japan are combined sewer systems that carry both untreated wastewater and rainwater simultaneously. Combined sewer systems pose a high risk of causing environmental contamination, especially during heavy rains and flooding events, which have become more pronounced because of climate change [
19]. However, there are no regulations limiting the discharge of antimicrobial resistance (AMR), and the actual amount of AMR is unknown, highlighting a potential gap in environmental monitoring and regulation.
AMR affects all countries, but the burden is higher in lower–middle-income countries (LMIC) such as Guatemala, Honduras, El Salvador, Panamá, Paraguay, Haiti, the Dominican Republic, Belize, Suriname, Uruguay, French Guiana, and Guyana because of various challenges. Studies have also mentioned that only 50–60% of Latin America is connected to sewage, and only 30% of domestic sewage is treated. A lack of financial resources in most countries poses challenges to the effective management of wastewater since it is one of the hot spots for AMR [
20].
Indian rivers contain antibiotic residues that may contribute to the growing problem of AMR and may have negative effects on the ecosystem and human health [
21,
22]. Untreated pharmaceutical wastewater and hospital effluents are a major source of contamination. One study mentioned that three antibiotics, ofloxacin, norfloxacin, and sulfamethoxazole, were detected in river water samples from four Indian rivers. They were detected and found to be two to five times higher than the safe limit.
Antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs) are highlighted as the most frequently reported pharmaceuticals in African waters. Examples of detected antibiotics include sulfamethoxazole, with concentrations reaching 53.8–56.6 μg/L in Kenya and Mozambique. Amoxicillin, another antibiotic, has shown concentrations ranging from 0.087 to 272.2 μg/L in Nigeria. The NSAID ibuprofen was detected at concentrations of up to 67.9 and 58.7 μg/L in Durban City and the Msunduzi River (KwaZulu-Natal, South Africa), respectively. An antiretroviral drug, lamivudine, has reached concentrations of up to 167 μg/L in surface water samples from Nairobi and Kisumu City, Kenya. In Asian countries, antibiotics have been detected at concentrations reaching 365.05 μg/L in surface water samples. Concentrations of other pharmaceuticals in Asian environmental waters are generally lower compared with those in African waters [
23].
Antibiotic usage has increased globally, and if no policy reforms are made, it is expected to reach 126 billion defined daily doses in 2030. Overall antibiotic pollution is shown in
Figure 1. A significant portion (30–90%) of antibiotics is released into the environment, posing a threat to ecosystems. Antibiotics have been found in various environmental compartments, with water being the most commonly reported. Examples of frequently detected antibiotics include fluoroquinolones, tetracyclines, sulfonamides, macrolides, and beta-lactams [
24]. Despite the substantial use of antibiotics in aquaculture, only a relatively small percentage (20~30%) is absorbed by the aquaculture products themselves. This low utilization rate suggests that a significant portion of antibiotics may not serve their intended purpose within the target organisms [
25]. Hence, the use of antibiotics in both terrestrial and aquatic animal production is contributing to ABR, which is a major global health challenge. The presence of antibiotics in different environments depends on their physicochemical properties, including the octanol/water dividing coefficient (Kow), the distribution coefficient (Kd), separation constants (pKa), vapor pressure, and Henry’s law constant (KH). Stability and decomposition rates vary among different antibiotics [
26]. For example, penicillin is easily decomposed, while fluoroquinolones and tetracyclines are more stable, leading to longer persistence and potential accumulation in the environment. Beta-lactam antibiotics, like penicillin, have beta-lactam rings that contribute to their degradation in the environment. In contrast, ciprofloxacin and erythromycin are more resistant to degradation because of the absence of beta-lactam in their structures. Fluoroquinolones and sulfonamides are highlighted as potentially dangerous antibiotics in the environment, but they may undergo degradation when exposed to sunlight [
27,
28].
Figure 1. Antibiotic pollution in several countries.
In aquaculture, antibiotic use is driven by production intensification and the increasing incidence of aquatic animal pathogens. Prolonged antibiotic use in aquaculture has led to ABR among humans [
29,
30] since many antibiotics used in aquaculture, such as tetracycline, macrolides, and aminoglycosides, are critically important for human treatment according to the World Health Organization (WHO). The 2022 Global Antimicrobial Resistance and Use Surveillance System (GLASS) report reveals alarming resistance rates among prevalent bacterial pathogens worldwide. Infections from resistant bacteria kill 700,000 people every year, with over 90% of them in low- and middle-income countries. Key findings include a median reported rate of 42% for third-generation cephalosporin-resistant
Escherichia coli (
E. coli) and 35% for methicillin-resistant
Staphylococcus aureus (MRSA) in 76 countries [
31,
32]. Urinary tract infections caused by
E. coli show reduced susceptibility to standard antibiotics, posing challenges in treatment.
Klebsiella pneumoniae (
K. pneumoniae) exhibits elevated resistance levels against critical antibiotics, potentially leading to the increased use of last-resort drugs like carbapenems. Projections indicate a twofold surge in resistance to last-resort antibiotics by 2035. The urgent need for robust antimicrobial stewardship practices and enhanced global surveillance is underscored to address the growing threat of antibiotic resistance. Additionally, by 2050, ABR will be accountable for 10 million deaths annually and harm the economy in a manner similar to that of the global financial crisis of 2008–2009 [
33].
Meanwhile, ABR in Southeast Asia (SEA) aquaculture involves 17 different drug classes, with the most commonly reported resistances occurring in aminoglycosides, beta-lactams, (fluoro)quinolones, tetracycline, sulfa groups, and multi-drug resistances.
E. coli,
Aeromonas, and
Vibrio spp. are the most commonly reported bacteria resistant to antibiotics in SEA aquaculture [
34]. Recent studies have identified antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes (ARGs) in various environments impacted by human activities. Overuse of antibiotics in fish farming exerts selective pressure on bacteria, favoring the development of antibiotic-resistant strains. Diverse bacteria, naturally resistant or acquiring resistance, have been detected in water samples from wastewater, recreational areas, surface waters, and drinking sources [
35]. For instance, drinking water distribution systems, which include pipelines and water reservoirs, are susceptible to biofilm formation. Biofilms in these structures can harbor ARBs and ARGs, contributing to drinking water contamination. One study observed, through high-throughput quantitative polymerase chain reaction (PCR), about 285 ARGs, especially
sul1,
ermB,
tetQ,
tetW,
cfr,
cmlA,
fexA,
fexB,
floR, and
qnrS, as well as mobile genetic elements (MGEs), in water samples from drinking water treatment plants located at Hangzhou City, eastern China [
36]. Another study detected MGEs, such as transposases and
intI-1 genes, suggesting their critical role in antibiotic resistance dissemination in drinking water. Sediment samples from water reservoirs located in central China have revealed the presence of 174 ARGs, with multidrug, sulfonamide, and vancomycin ARGs being the most prevalent. MGEs are identified as the main biotic factors contributing to ARG dissemination in sediment [
37]. Similarly, most borehole and tap water samples in Ghana show no
E. coli counts, and over 50% show no detectable
P. aeruginosa. However, over half of the
E. coli isolates were multidrug-resistant (MDR).
E. coli isolates have shown high resistance to cefuroxime, trimethoprim–sulphamethoxazole, and amoxicillin–clavulanate [
38].
E. coli from French drinking water with the
blaCTX-M-1 gene in an IncI1/ST3 plasmid demonstrates the presence of resistant bacteria and a specific resistance mechanism in a real-world setting. Linking the
blaCTX-M-1 gene found in
E. coli to
K. pneumoniae outbreaks and its presence in animal
E. coli highlights the potential for cross-species transmission and environmental reservoirs. Significant differences have been observed in the structure of bacterial communities and the profiles of ARGs and MGEs between summer and winter samples from the Douhe Reservoir, China. Six specific MGE subtypes were identified as crucial for the spread of ARGs in both water and sediment. The evolution of bacterial communities appears to be the primary driver of changes in ARGs. In addition, one study also mentioned that environmental factors, particularly temperature, nitrates, total dissolved nitrogen, and total phosphorus, showed significant correlations with variations in bacterial communities, ARGs, and MGEs [
39].
On the other hand, our aquatic environments are also under siege by a torrent of pollutants: industrial and agricultural runoff, municipal wastewater, oil spills, plastic waste, toxic chemicals, excess nutrients, sedimentation, radioactive materials, and mercury (Hg) contamination. These contaminants wreak havoc on aquatic ecosystems, killing species, disrupting food webs, and leaving water quality in a perilous state [
40]. They also pose risks to human health through waterborne diseases and contaminated food sources. The increase in aquatic environment pollution has become a concerning and unfortunately common trend [
41]. Human activities, including industrialization, agriculture, urbanization, and improper waste disposal, have led to the widespread contamination of water bodies, making water pollution a persistent and pervasive issue [
42]. The rise in aquatic environment pollution potentially contribute to an increase in ABR, posing a significant public health concern [
43]. One study found evidence showing the long-term impact of Hg contamination on increases in the persistence of environmental ARGs, specifically for tetracycline, sulfonamides, and trimethoprim. Interestingly, agriculturally important bacterial groups like Nitrospirae did not recover in the contaminated soils, suggesting the complex interplay between metal chemistries, especially Hg, soil pH, ABR, and microbial communities [
44]. The widespread use of plastics also leads to the presence of ARGs in water bodies. Microplastics provide a perfect vector for microbes to colonize and exchange ARGs through a process called horizontal gene transfer (HGT) [
45]. A previous study mentioned that aquatic environments serve as reservoirs for diverse bacterial populations, creating ideal conditions for the exchange and transfer of genetic material containing resistance genes through selective pressure that occurs when antibiotics are present in the environment [
46]. This exposure creates conditions where bacteria are forced to develop ABR or mechanisms to survive and multiply, while susceptible bacteria are eliminated. The continued overuse or presence of antibiotics sustains this selective pressure, allowing resistant strains to thrive and become dominant [
47]. On the other hand, HGT is a crucial process, facilitating the rapid spread of resistance genes among bacteria. Through HGT mechanisms such as conjugation, transformation, and transduction, resistant bacteria can transfer genetic material containing resistance traits to other bacteria, even those of different species [
48]. This exchange enables the swift dissemination of resistance genes throughout bacterial populations, contributing significantly to the widespread occurrence of antibiotic resistance. The interconnectedness of bacterial populations in aquatic environments facilitates this gene exchange, making it vital to monitor and understand these ecosystems to effectively combat ABR [
49].
The occurrence of ABR in aquatic environments poses a significant threat not only to human health but also to a wide range of ecological and environmental aspects, including ecosystems and food webs, disrupting their delicate balance and resilience. When the delicate balance of microbial communities is thrown off, both nutrient cycling and ecosystem stability suffer. This disruption, often fueled by the rise of antibiotic-resistant bacteria, directly impacts the organisms within these systems, posing a major threat to their well-being [
50]. This disruption can extend to soil ecosystems, affecting the intricate relationships between microorganisms and plants, thus influencing agricultural productivity and soil fertility. In agricultural settings, for instance, the use of antibiotics in livestock can foster the development of resistant strains that affect animal health, potentially impacting humans through the food chain [
51]. Similarly, in aquatic environments, the presence of antibiotic-resistant bacteria can disrupt the health of aquatic organisms, leading to imbalances in the ecosystem. The impact on fisheries and aquaculture due to antibiotic-resistant bacteria can affect food production and access to protein sources for humans [
52].
3. Potential Negative Effects of ABR in Aquatic Environments on Human and Animal Health
The increase in ABR in aquatic environments brings forth significant concerns for both human and animal health. It has a multifaceted impact on public health, food security, economies, food systems, and livelihoods. Antibiotic-resistant organisms, particularly bacteria, can infiltrate aquatic environments through a multitude of pathways, creating concerns for both the environment and public health [
53]. Once in the water, these antibiotic residues can affect the aquatic microbiome, which includes the diverse communities of microorganisms in the water [
54]. Alterations to the environmental microbiome can have cascading effects on ecosystem health, potentially disrupting the balance of microorganisms and other organisms within the aquatic ecosystem. This can lead to the transmission of resistant bacteria to humans and animals through various pathways, such as contaminated water sources and the consumption of seafood [
55]. The potential for infections that are challenging to treat with conventional antibiotics poses a serious threat to public health. Moreover, the interconnected nature of the food chain means that bacteria in aquatic organisms may be transferred to humans, further increasing the risk of multidrug-resistant infections. The persistence of antibiotic residues and resistant bacteria in aquatic environments not only threatens the effectiveness of medical treatments but also raises concerns about their long-term impact on ecosystem health and the potential emergence of novel resistance mechanisms [
56]. ABR from animals can reach humans through various modes of transmission, including the food chain, handling, processing, transport, storage, and the preparation of food products. The transmission can begin at the farm level and spread within and between communities. Inadequate antibiotic use is linked to the decreased ability of fish species to metabolize drugs effectively, leading to prolonged antibiotic residues in fish meat [
57]. These residues can persist in the terrestrial ecosystem through the food chain, and an estimated 70–80% of active compounds are eliminated through feces, contributing to antibiotic dispersion in wastewater and influencing diverse ecosystems. The existence of ARGs in aquaculture environments increases the risk of human and animal exposure to bacteria carrying these resistance traits. If these resistant bacteria are transmitted to humans through the consumption of contaminated aquaculture products or direct contact, it can lead to infections that are challenging to treat with common antibiotics [
58].
Aquaculture settings where antimicrobials are used may act as reservoirs for antimicrobial resistance genes. This means that bacteria within the aquaculture environment exposed to antimicrobials may develop resistance to these drugs [
59]. The presence of antimicrobial resistance genes in aquaculture settings raises concerns about the potential transmission of resistant bacteria to humans and animals, posing a threat to public health. In addition, the occurrence of ABR in aquatic environments has also led to changes in the aquatic environment’s microbiome, which can have broader implications for ecosystem health and function. The microbiome plays a crucial role in nutrient cycling, biodiversity maintenance, carbon sequestration, and freshwater availability. Disruptions to these ecosystem functions can have cascading effects on the overall health and sustainability of aquatic ecosystems [
60]. ABR also poses a significant threat to veterinary medicine, jeopardizing animal production and, consequently, food security. The World Bank projects a potential 11 percent decline in livestock production in low-income countries by 2050 due to the challenges presented by ABR. This projection suggests a substantial loss of nearly 4 percent of the world’s annual gross domestic product (GDP) by 2050 due to AMR [
61]. Despite efforts, the levels and patterns of antimicrobial use in aquaculture globally are largely undocumented, limiting the development of targeted interventions and policies for antimicrobial stewardship.
On the other hand, since the advent of penicillin in the mid-20th century, antimicrobial treatments have played a crucial role not only in human medicine but also in veterinary care. Apart from therapeutic and prophylactic uses, low doses of antimicrobials have been added to animal feed to promote faster growth. While an increasing number of countries prohibit the use of antimicrobials as growth promoters, it remains a common practice in many parts of the world. However, the growth-promoting use of antibiotics among animals is associated with drawbacks. It does not result in the irreversible destruction of harmful bacteria, and sublethal doses act as selective pressure, promoting the evolution of bacterial strains resistant to antibiotics. This poses threats not only to human health but also to animal health, welfare, and sustainable livestock production, with implications for food security and people’s livelihoods [
62]. Antibiotics, including macrolides and sulfonamides, have been found to negatively affect the growth, development, and reproduction of aquatic organisms such as algae. Antibiotics can also damage the photosystems of plant cells and interfere with carbon dioxide transformation [
63].
4. Summary
The aquatic environment is a vital ecosystem encompassing oceans, seas, lakes, and rivers, crucial for sustaining diverse species and providing essential ecosystem services. However, the rampant use of antibiotics in aquaculture and widespread human activities have led to the emergence of ABR within various antibiotic classes. Better understanding of ABR and its impacts on aquatic environments is crucial to deciphering microbial interactions, comprehending antibiotic resistance dissemination, and managing the ecological impact of microbial communities. A future-oriented perspective on addressing ABR in aquatic environments involves advancing scientific knowledge, fostering collaboration among stakeholders, implementing stringent regulations, and raising public awareness. By embracing these approaches, we can work toward effectively mitigating the spread of ABR and safeguarding the health of aquatic ecosystems and public well-being.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25063080